Keywords
Earwax, Cerumen, Cerumenolytic, Cerumen impaction
Earwax, Cerumen, Cerumenolytic, Cerumen impaction
The excess accumulation of cerumen (earwax) is a common cause for patients to seek treatment by a general physician, family physician, or otolaryngologist1. At least 8 million ear irrigations are performed each year for this condition2. Cerumen impaction is estimated to affect between 2 and 6% of the general population in the United States. As many as 65% of individuals over 65 years of age and up to 36% of those with mental retardation experience cerumen impactions2–5.
Cerumen impaction has important clinical implications in terms of the general well-being of patients and may be associated with temporary hearing loss, pain, itching, tinnitus, external otitis, vertigo, and even chronic cough5. Cerumen impaction can temporarily decrease hearing acuity by as much as 45 dB6. For the elderly, this hearing impairment can have a negative impact on quality of life by causing difficulties with communication, cognition, social isolation, anxiety, depression, and even physical mobility1,7,8. All too often, decreased hearing with advancing age, either gradual or acute, is perceived by the patients and/or their caregivers as a natural, almost expected, phenomenon, which does not warrant intervention1. However, studies have shown that hearing is significantly improved following the removal of impacted cerumen4,9.
There are currently several commercially available cerumen removal products. These products include oil-based (e.g., almond oil), water-based (e.g., acetic acid), and non-water, and non-oil-based (e.g., propylene glycol) preparations10. Unfortunately, these preparations are minimally effective at disintegrating cerumen impactions and often require multiple doses per day over several days to achieve satisfactory results11,12.
None of the agents that are currently available has shown a clear advantage in terms of efficacy in removing cerumen2,10,13,14. Previous studies have found that these products are often less effective or no better than deionized water12,15. Moreover, they typically clear cerumen less than half of the time10,16. Systemic reviews have found no topical cerumenolytic clearly superior to any other or to saline or sterile water10,13,14.
These results have prompted the search for a better cerumenolytic agent, and we have identified ingredients that could quickly, effectively, and safely breakdown or dissolve human cerumen when combined. Consequently, a new product has been developed, which benefits from a dual-action mechanism for breaking down human cerumen. The current study compared the new cerumen removal product (EOS-002) with two commercially available products for their ability to breakdown or disintegrate samples of human cerumen in vitro.
Institutional Board Approval of the University of North Texas Health Science Center (UNTHSC) and patient informed consent were obtained prior to commencement of this study.
Human cerumen samples (approximately 30 to 50 μg each) were placed in 10 x 75 mm polypropylene test tubes at room temperature. The samples were taken without restriction in terms of patient characteristics. The physician utilized a curette to remove the cerumen from the subjects outer ear canal. The samples were placed in small plastic storage tubes with lids, labeled with date of extraction along with a general description of the physical characteristics (dry, wet or mixed). The samples were required to be at least 30 μg in size. Approximately 1 mL of each test solution was added to each test tube, and the samples were incubated at room temperature for 30 minutes, with grading recorded at 5, 10, 15 and 30 minutes. Photographs were taken for representative samples at 2.5-minute intervals. Each comparison for each time point was performed in replicate tubes (n = 24 or 19). The sample size was driven by the availability of subjects willing to participate in the collection trial. A total of 86 cerumen samples were available during the duration of the testing. The comparative products were used as controls, as these products are well recognized by physicians and consumers.
The samples were graded at 5 minute and 10 minute time points, without moving the tubes. However, the test tubes were shaken at the 15 minute and 30 minute time points to simulate the rinse procedure that would normally occur in the clinical use setting.
The test solutions were as follows:
• Solution 1 - Glycolic acid/bicarbonate formulation (EOS-002; Eosera Inc., Fort Worth, TX; 2016)
• Solution 2 - Carbamide peroxide 6.5% (Debrox, Prestige Brands, Tarrytown, NY; 2016)
• Solution 3 - Carbamide peroxide 6.5% (Murine Earwax Removal System, Prestige Brands, Tarrytown, NY; 2016)17
A grader (affiliated with the sponsor company) was blinded as to the identity of the test solutions assessed the disintegration (breakdown) of cerumen at 5, 10, 15, and 30 minutes. A 5-point disintegration grading scale was developed for assessing the effects of different formulations on human cerumen (Table 1). This grading scale was adapted from those of Jimenez et al.18 and Fraser19.
Means and standard deviations were calculated for each treatment group at the 5, 10, 15, and 30 minute time points. Between-group comparisons were performed using Student’s t test. A P value of ≤ 0.05 denoted a statistically significant difference between treatment groups. Statistical analysis was conducted with Microsoft Excel for Mac 2011, version 14.6.0.
For the comparison between EOS-002 and Solution 2, 24 samples each were available for each time point. The time course found significant differences between EOS-002 and Solution 2 (P < 0.0001) in grading scores at all time points (5 min, 10 min, 15 min, and 30 min) (Figure 1). The mean disintegration scores at 5 minutes were 1.63 ± 0.7 for EOS-002 and 0 ± 0 for Solution 2. No sample out of the 24 samples in the Solution 2 group had a score above 0 at 5 minutes compared with 24 out of 24 for EOS-002 (range 1 to 3).
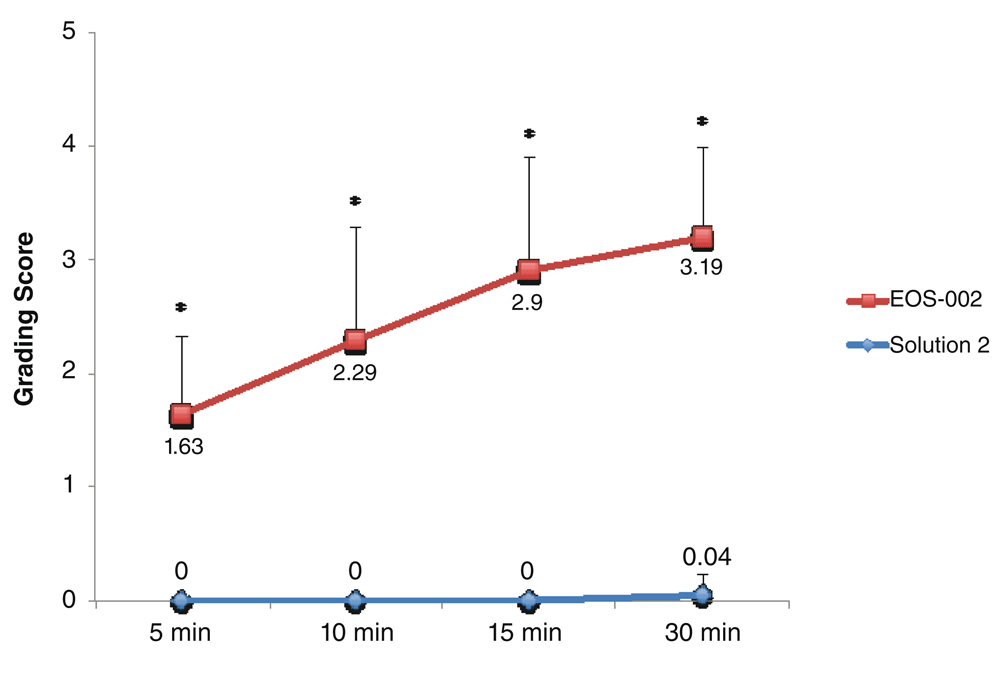
All incubations were performed at room temperature. *P < 0.0001.
For the evaluations of EOS-002 and Solution 3, 19 samples each were available for each time point. Similarly, the time course demonstrated significant differences between EOS-002 and Solution 3 (P < 0.0001) in grading scores at all time points (Figure 2). The mean disintegration scores at 5 minutes were 1.68 ± 1.0 for EOS-002 and 0.05 ± 0.2 for Solution 2. Only 1 out of 19 samples in the Solution 2 group had a score above 0 (1) at 5 minutes, compared with 16 out of 19 samples for EOS-002 (range 0 to 3).
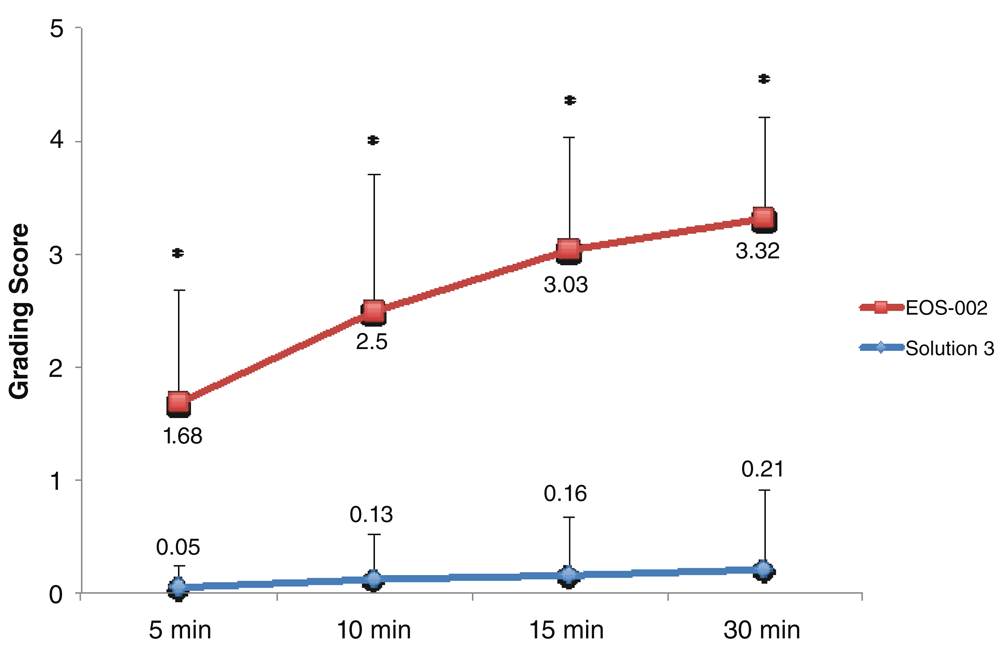
All incubations were performed at room temperature. *P < 0.0001.
When the data for both comparisons were combined, the mean disintegration scores at 10 minutes were 2.38 ± 1.1 for the EOS-002-treated samples and 0.06 ± 0.3 for the carbamide peroxide 6.5%-treated samples (n = 43 for both groups; Figure 3). As expected, all time points showed significant differences in favor of EOS-002 in terms of the disintegration scores.
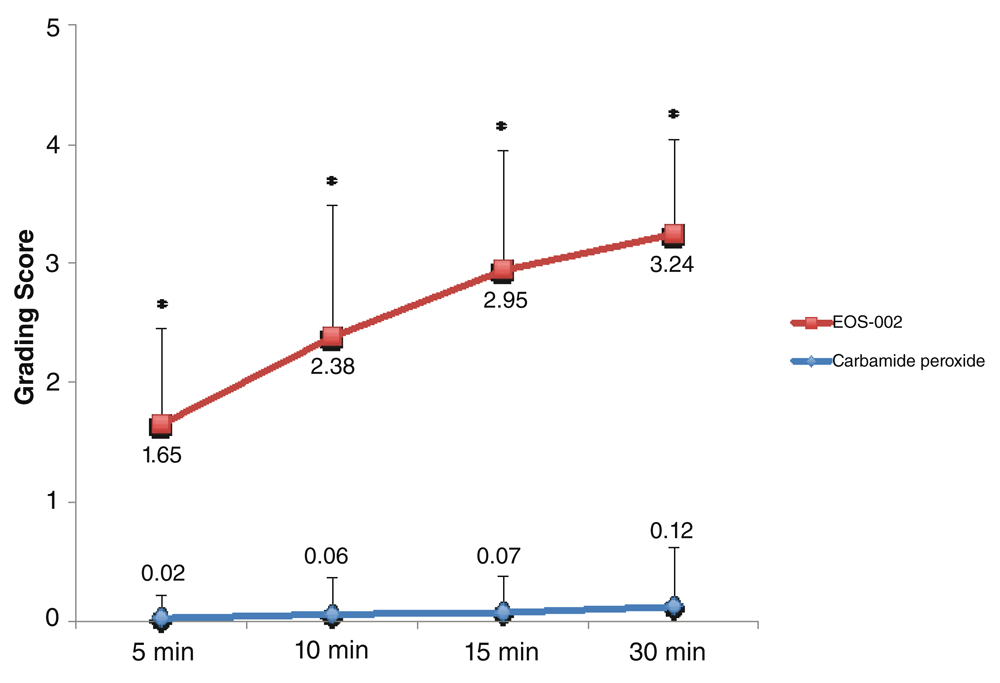
All performed were conducted at room temperature. *P < 0.0001.
For the comparison between EOS-002 and Solution 2, the cerumen samples started to swell and disintegrate within 2.5 minutes of exposure to EOS-002 (Figure 4). At 15 minutes, these samples were noticeable disrupted and dispersed compared with their appearance prior to treatment. However, after 15 minutes of exposure to Solution 2, there was no discernable change to the samples.
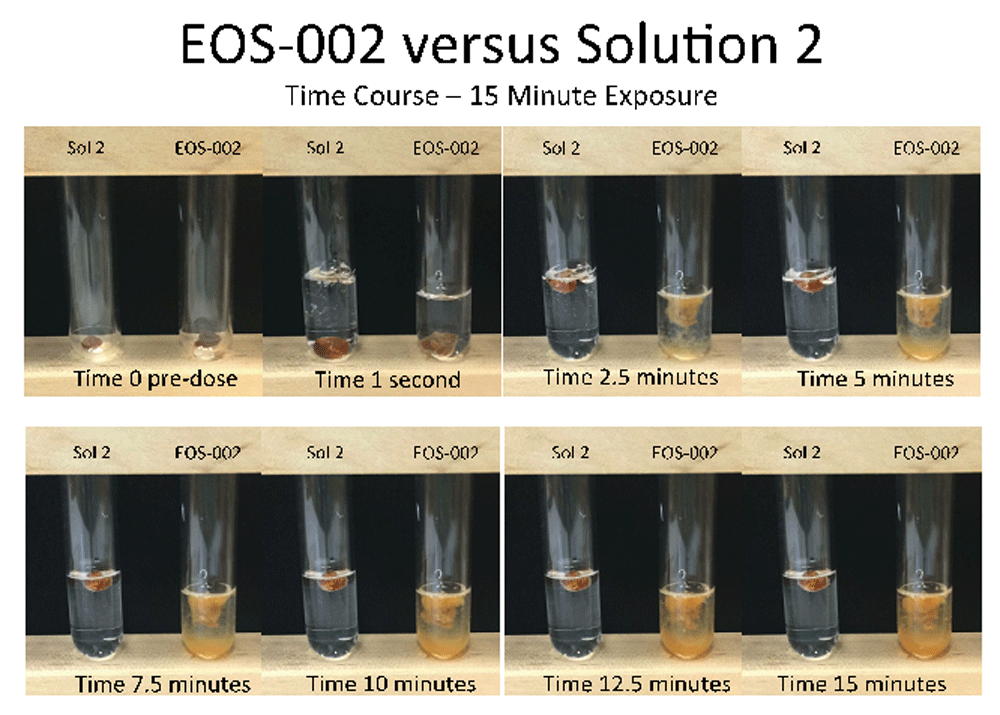
All incubations were performed at room temperature.
As with the above experiments, for the evaluations of EOS-002 and Solution 3, within 2.5 minutes of exposure to EOS-002, the cerumen samples started to swell and disintegrate (Figure 5). At 15 minutes, the EOS-002 sample was noticeable disrupted and dispersed compared with its appearance before treatment. However, after 15 minutes of exposure to Solution 3, there was little to no change to the sample.
Both photographic records and the time course studies for disintegration scores demonstrated that EOS-002 was effective at quickly breaking down human cerumen under room temperature conditions. Samples incubated in EOS-002 demonstrated significantly higher disintegration scores than the two comparators at every time point measured (P < 0.0001). From the photographic studies, differences between EOS-002 and the other two products could be seen within 2.5 minutes. Differences in disintegration scores were also observed within 5 minutes (the earliest graded time point). Only a small amount of disintegration was observed for the samples exposed to the 2 products containing carbamide peroxide 6.5%, even after 30 minutes.
An in vitro study, conducted by Saxby et al.15, evaluated the cerumenolytic activity of 6 different preparations (distilled water; olive oil; sodium bicarbonate 5%, dexamethasone 0.05% + framycetin sulphate 0.5% + gramicidin 0.005% [Sofradex, Sanofi-Aventis, Guildford, UK]; urea + hydrogen peroxide 5% in glycerol; and bethamethasone sodium phosphate 0.1% [Vistamethasone, Cardinal Health Martindale Products, Brentwood, UK]). Each cerumen sample (5 mm in diameter and 3 mm thick) was placed into a test tube that contained 5 mL of one of the test solutions and allowed to incubate at room temperature. At 30 minutes of exposure, the aqueous-based solutions had caused a slight amount of disintegration, while the oil-based solutions (olive oil or urea + hydrogen peroxide) produced no visible change to the cerumen samples (Table 2). Distilled water and sodium bicarbonate 5% produced the greatest amounts of disintegration. It should be noted that it might not be feasible for a patient to treat their ears with a cerumenolytic for 30 minutes prior to irrigation. The current study suggests substantial disintegration of cerumen might be possible in as little as 5 minutes of exposure with the novel glycolic acid/bicarbonate formulation.
1Grading scale adapted from Fraser et al., 1970, and Jimenez et al., 2008. Grade 0 = no change; Grade 1 = slight disintegration; Grade 2 = moderate disintegration; Grade 3 = substantial disintegration; Grade 4 = complete disintegration.
2 – = no visible change; + = slight disintegration; ++ = partial disintegration; +++ = substantial disintegration.
3 – = no visible change; + = coloration of the agent; ++ = slight disintegration; +++ = partial disintegration; ++++ = substantial disintegration; +++++ = complete disintegration.
4 – = no visible change; + = slight solvent effect; ++ = partial disintegration; +++ = complete disintegration.
Min = minutes; h = hours; d = days
| 5 min | 10 min | 15 min | 30 min | 1 h | 2 h | 3 h | 12 h | 3 d | |
|---|---|---|---|---|---|---|---|---|---|
| Current study1,2 (Performed in test tubes at room temperature) | |||||||||
| EOS-002 | 1.65 | 2.38 | 2.95 | 3.24 | |||||
| Carbamide peroxide 6.5% | 0.02 | 0.06 | 0.07 | 0.12 | |||||
| Saxby et al., 20132 (Performed in centrifuge tubes at room temperature) | |||||||||
| Distilled water | + | ++ | +++ | ||||||
| Olive oil | - | - | - | ||||||
| Sodium bicarbonate | + | ++ | +++ | ||||||
| Dexamethasone 0.05%/framycetin sulphate 0.5%/gramicidin 0.005% | + | ++ | ++ | ||||||
| Urea + hydrogen peroxide | - | - | - | ||||||
| Betamethasone sodium phosphate 0.1% | + | ++ | ++ | ||||||
| Bellini et al., 19893 (Performed in centrifuge tubes at 36.4°C) | |||||||||
| Arachis oil base containing 10 % oil of terebinth (turpentine) and dichlorobenzene, chlorbutol, and benzocaine | - | + | + | + | |||||
| Dioctyl sodium sulphosuccinate | ++ | ++++ | ++++ | +++++ | |||||
| Earex | - | + | + | + | |||||
| Stores Own | ++ | +++ | ++++ | ++++ | |||||
| Olive oil | - | + | + | + | |||||
| Water | +++ | +++ | ++++ | +++++ | |||||
| Bicarbonate | + | ++ | ++ | ++ | |||||
| Bellini et al., 19893 (Performed in pasteur pipettes at 36.4°C) | |||||||||
| Arachis oil base containing 10 % oil of terebinth (turpentine) and dichlorobenzene, chlorbutol, and benzocaine | - | - | + | + | |||||
| Dioctyl sodium sulphosuccinate | +++ | ++++ | +++++ | +++++ | |||||
| Earex | - | - | + | + | |||||
| Acetone | - | - | - | + | |||||
| Olive oil | + | + | + | + | |||||
| Water | +++ | ++++ | ++++ | +++++ | |||||
| Water (2) | ++ | +++ | ++++ | +++++ | |||||
| Bicarbonate | + | ++ | ++ | +++ | |||||
| Fraser et al., 19704 (Performed in test tubes at 37°C) | |||||||||
| Arachis oil base containing 10 % oil of terebinth (turpentine) and dichlorobenzene, chlorbutol, and benzocaine | - | - | - | ||||||
| Dioctyl sodium sulphosuccinate | - | + | +++ | ||||||
| Olive oil | - | - | - | ||||||
| Sodium bicarbonate | - | - | +++ | ||||||
| Triethanolamine polypeptide oleate 10% in propylene glycol | - | - | ++ | ||||||
| Dioctyl sodium sulphosuccinate in a corn oil base (ear capsules) | - | - | - | ||||||
Bellini et al.20 performed an in vitro study on eight different preparations (Waxsol, dioctyl sodium sulphosuccinate 0.5% in a water-miscible base; Cerumol, paradichlorobenzene 2%, chlorbutol 5%, and turpentine oil 10%; Earex, arachis oil 33.3% v/v, almond oil 33.3% v/v, rectified camphor oil 33.3% v/v; dioctyl sodium sulpho-succinate 5% w/v; olive oil; sodium bicarbonate; distilled water; and acetone). The tubes containing the samples (40 mg) and test solutions (0.5 mL) were incubated at 36.4˚C for up to 2 hours in either pasteur pipettes (Series 1) or plastic centrifuge tubes (Series 2) (Table 2). Bellini and colleagues found a modest amount of cerumenolytic activity with sodium bicarbonate, a component of the EOS-002 formulation. No changes were observed at 15 minutes for Earex and the preparations containing arachis oil, and olive oil. Conversely, the present study found moderate to substantial disintegration of cerumen with EOS-002 at 15 minutes at room temperature. One might expect even more rapid disintegration with this formulation at body temperatures.
Fraser and colleagues19 also conducted their studies of different cerumenolytic preparations in test tubes incubated at 37˚C for up to 3 days. Interestingly, they found no visible change with any of the preparations at 15 minutes (Table 2). Contrast this with the cerumen samples in the current study exposed to EOS-002, which showed observable disintegration within 5 minutes.
Another in vitro study, by Uppal et al.21 compared 5 ear drop formulations (5% NaHCO3, 3% H2O2, dexamethasone sodium metasulphobenzoate 0.05%, framycetin sulphate 0.5%, gramicidin 0.005%), 0.33% acetic acid and 0.9% NaCl) for clearing grommets blocked with freshly harvested thick middle ear effusion fluid. These grommets were housed in models of the ear canal constructed using 2 mL syringes. In the intervention groups five drops of each formulation were instilled into each syringe 3 times a day for a total of 7 days. The number of grommets cleared ranged from 1.3% (no drops) to 36.7% (29/79; 5% NaHCO3). Acetic acid 0.33% produced clearing in 27.2% (22/81) of the grommets. The glycolic acid/bicarbonate formulation of EOS-002 has similar but enhanced characteristics compared with some of the most effective eardrops tested in the Uppal et al. study21.
Another in vitro study evaluated a liquid enzyme-based cerumenolytic formulation18. Samples of cerumen (30 mg) were incubated in glass test tubes at 37˚C without agitation. After 5 minutes of exposure, there was evidence of disintegration with the enzyme-based formulation. However, at 30 minutes, there was almost no qualitative change in the samples exposed to the commercial formulations, one of which was the same product as Solution 3 in the current study. The results of the current study corroborate this previous observation. Little change to the samples were observed after 15 minutes with Solution 3.
It is proposed that EOS-002 uses a dual-action mechanism to disintegrate human cerumen. Wax ester and fatty acid lipid components of the cerumen are disrupted by the bicarbonate system of the formulation22,23. This system breaks carboxylic acids down to their more water-soluble carboxylate salts. The glycolic acid system of the product chelates calcium ions from the calcium-dependent cell adhesion molecules resulting in the disruption of cadhedrins, which allows the cells of the keratin sheet to break apart24,25. It is feasible that the glycolic acid also works in conjunction with a osmolarity variance between the formulation and the keratinocytes, leading to an influx of water into the cells leading to swelling and disruption of the wax mass26.
The current study is limited by its in vitro design. The incubations were conducted at room temperature and results could vary at body temperatures in vivo. These results should be confirmed in a prospective randomized clinical study.
Overall, evidence from the literature suggests aqueous preparations are better for disrupting human cerumen than oil-based preparations15,19,20. Furthermore, bicarbonate formulations have demonstrated efficacy for causing the disintegration of cerumen in vitro. Another study showed that an acidic preparation had moderate efficacy in breaking down cerumen in vitro21. These findings support the results of the current study, which demonstrated the rapid disintegration of cerumen in sample exposed to EOS-002 comprising a glycolic acid/bicarbonate formulation. Conversely, two commercially available products, both containing carbamide peroxide 6.5%, had minimal effects on the cerumen samples. The in vitro results with EOS-002 are promising. A small exploratory study in humans has recently been performed, which demonstrated efficacy of the product in disintegrating cerumen, in order to aid in the removal of impactions (unpublished study; Fullington, D, Song, J, Gilles, A, Guo, X, Hua, W, Anderson, C, Griffin, J).
Dataset 1: Raw data for Figure 1. EOS-002 vs Solution 2. DOI, 10.5256/f1000research.10279.d14437327.
Dataset 2: Raw data for Figure 2. EOS-002 vs Solution 3. DOI, 10.5256/f1000research.10279.d14437428.
Dataset 3: Raw data for Figure 3. EOS-002 vs combined data from Solutions 2 & 3. DOI, 10.5256/f1000research.10279.d14437529.
Institutional Board Approval of the University of North Texas Health Science Center (UNTHSC IRB Project # 2015-114) and patient informed consent were obtained prior to commencement of this study.
JG and CA conceived, designed the study and carried out the research with consultation from JK and BH. JG prepared the first draft of the manuscript. CA and WD contributed to the experimental design, discussion and mechanism of action sections of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
JK, BH, and WD declare no conflict of interest. CA was a temporary employee of the sponsoring company (Eosera, Inc.) during the development of the product, was provided fair market compensation during his employment, and is an author on the pending patent application. JG is an employee and equity holder in Eosera, Inc., and is an author on the pending patent application.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 29 Apr 25 |
read | ||
|
Version 1 29 Nov 16 |
read | read | |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)