Keywords
Sphingosine kinase 2; antibody validation; immunoblotting, immunoprecipitation, immunofluorescence
This article is included in the Antibody Validations gateway.
Sphingosine kinase 2; antibody validation; immunoblotting, immunoprecipitation, immunofluorescence
Following the reviewers’ comments, this revised version of the manuscript now includes additional comments in the Conclusion section addressing the low expression of SK2 protein in many cell lines, which may impact on immunoblotting and immunofluorescence detection. It also includes further discussion on the splice isoforms of human SK2.
See the authors' detailed response to the review by Maria Laura Allende
See the authors' detailed response to the review by Dagmar Meyer Zu Heringdorf
Sphingolipids are an important family of cellular molecules that form critical structural components of cell membranes, as well as performing numerous signaling functions1. Of the many enzymes responsible for the biosynthesis and metabolism of sphingolipids, the sphingosine kinases (SKs) are of particular interest to study as they catalyze the formation of sphingosine 1-phosphate (S1P), and in doing so can promote cell survival, proliferation, migration and angiogenesis2. Both sphingosine kinases, SK1 and SK2, have been shown to be upregulated in various human cancers and both have documented roles in mediating oncogenesis3,4. However, where SK1 and its roles in cancer development are relatively well characterized, SK2 remains somewhat enigmatic as, in addition to the pro-cancer functions it shares with SK1, SK2 can also facilitate cell cycle arrest and cell death5,6.
SK2 is ubiquitously expressed in all cells and tissues, but is expressed most highly in the liver, kidney and brain7. At the mitochondria, SK2-generated S1P has been proposed to facilitate the activation of Bak and subsequent mitochondrial membrane permeabilisation and cytochrome c release5. Notably, SK2 can also function as an epigenetic regulator, where S1P produced by nuclear-localized SK2 can inhibit the activity of histone deacetylases 1/2 resulting in increased transcription of specific genes, such as cyclin-dependent kinase inhibitor p21 and transcriptional regulator c-fos8. As SK1 does not appear to localize as prominently to these internal organelles, it is believed that the subcellular localization of SK2 is critical for the additional functions it performs. However, the mechanisms regulating the localization and functions of SK2, allowing it to switch between pro-apoptotic and pro-survival under certain conditions, remain poorly understood.
In order to study SK2 and better characterize its roles in normal cells as well as in cancer, reliable and properly validated tools are required. Antibody-based methods, such as immunoblotting (IB), immunoprecipitation (IP) and immunofluorescence (IF), are particularly useful as tools to examine and visualize important aspects of SK2 biology, like subcellular localization, expression and interaction with regulatory proteins. A number of groups in the field have taken to generating their own in-house SK2-specific polyclonal antibodies9,10, but to our knowledge there has been no systematic validation of commercially available SK2 antibodies. Here, we compare two commercially available SK2 antibodies, and validate the suitability of their use in IB, IP and IF using various human and mouse cells lines. We have examined a rabbit polyclonal SK2 antibody from Proteintech, which is raised against amino acids 266–618 of recombinant human SK2a, and a rabbit polyclonal SK2 antibody from ECM Biosciences, which is raised against a synthetic peptide corresponding to amino acids 36–52 of human SK2a. The Proteintech SK2 antibody has been previously utilized in one publication for IB11, and the ECM Biosciences SK2 antibody has been used in multiple publications for IB12–15 and for IF16.
The following SK2 antibodies were assessed: rabbit polyclonal anti-SK2 (ECM Biosciences; anti-Sphingosine Kinase 2 (N-terminal region); #SP4621, lot #1) and rabbit polyclonal anti-SK2 (Proteintech Group, Inc; anti-SPHK2; #17096-1-AP, lot #00010361). The ECM Biosciences SK2 antibody was raised against a synthetic peptide coupled to keyhole limpet hemocyanin (KLH), corresponding to amino acids 36–52 of human SK2a, and was affinity purified with the SK2 peptide (without KLH). It is reported by the manufacturer to have cross-reactivity with rat and mouse SK2 [human, mouse and rat SK2 share 100% sequence identity in this region (determined using the align tool and protein sequences from www.uniprot.org)], and has been assessed by the manufacturer for use in IB and enzyme-linked immunosorbent assay (ELISA). The Proteintech SK2 antibody was raised against truncated recombinant GST-tagged human SK2a (amino acid residues 266–618 generated in Escherichia coli using the PGEX-4T plasmid). The SK2 target antibodies were then affinity purified using 6xHis-tagged antigen protein (to remove GST-specific antibodies) and then again with the immunising GST-tagged antigen protein. It is reported to have cross-reactivity with rat and mouse SK2 [80.2% sequence identity between human and mouse SK2, and 80.2% sequence identity between human and rat SK2 in this region (determined using the align tool and protein sequences from www.uniprot.org)], and according to the manufacturer can be employed for IB, ELISA, IP and immunohistochemistry. Mouse anti-α-tubulin (DM1A; Abcam; #ab7291) is a mouse monoclonal antibody, which was used as a loading control for IB analyses, at a dilution of 1:5,000. All antibody details, including information for secondary antibodies used, are provided in Table 1.
Human embryonic kidney (HEK) 293 cells (CellBank Australia; #85120602) and HeLa human cervical cancer cells (ATCC; #CCL-2) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Thermo Fisher Scientific Inc.), containing 10% heat-inactivated fetal bovine serum (FBS; Bovagen), 1 mM HEPES, penicillin (1.2 mg/ml) and streptomycin (1.6 mg/ml). Cells were grown at 37°C with 5% CO2 in a humidified incubator. Primary mouse embryonic fibroblasts (MEFs) were generated from both wildtype (WT) C57/Bl6 and Sphk2-/- C57/Bl617 mouse embryos at 14.5 days post coitum. The fibroblasts were isolated and cultured as described above, except they were maintained at 37°C in a humidified atmosphere with 10% CO2.
siRNA-mediated knockdown of SK2 was performed using human SPHK2 siGENOME SMARTpool siRNA (Dharmacon), which targets the following sequences: CCACUGCCCUCACCUGUCU, GCUCCUCCAUGGCGAGUUU, GAGACGGGCUGCUCCAUGA, CAAGGCAGCUCUACACUCA. Cells were seeded and grown to a cell density of approximately 50%, and were then transfected with 30 nM (final concentration) of either human SK2 siRNA or siGENOME non-targeting siRNA control pool (Dharmacon), using Lipofectamine RNAiMAX (Life Technologies), as per the manufacturer’s protocol. Cells were incubated with the siRNA complexes at 37°C for 48 h.
Specific details for all reagents used can be found in Table 2. Cells were pelleted by centrifugation (400 × g, 5 min, 4°C) and washed in cold phosphate buffered saline (PBS). Cell pellets were resuspended in extraction buffer [EB; 50 mM Tris/HCl buffer (pH 7.4) containing 150 mM NaCl, 10% glycerol, 1 mM EDTA, 0.05% Triton X-100, 2 mM Na3VO4, 10 mM NaF, 10 mM β-glycerophosphate, 1 mM dithiothreitol (DTT) and protease inhibitor cocktail (Roche)], and lysed by bath sonication (4× 30 sec on/off). Lysates were clarified (17,000 × g, 15 min, 4°C) and equal amounts of protein [as determined by a Bradford protein assay (Bio-Rad Laboratories)] were mixed with 5× Laemmli sample buffer, boiled at 100°C for 5 min, and separated by SDS-PAGE on a Criterion™ XT Bis-Tris 4–12% gradient gel (Bio-Rad Laboratories). Proteins were then transferred to nitrocellulose membrane (Pall Life Sciences) at 400 mA for 1 h. Membranes were blocked with 5% skim milk in PBS containing 0.1% Triton X-100 (PBS-T) for 1 h at room temperature, with gentle rocking. Membranes were probed with rabbit anti-SK2 antibodies diluted in Signal Boost primary antibody diluent at 1:1,000 (ECM Biosciences: 1 µg/ml; Proteintech: 687 ng/ml) overnight at 4°C with gentle rocking. Alternatively, membranes were probed with mouse anti-α-tubulin antibody diluted in 5% skim milk in PBS-T at 1:5,000 (200 ng/ml) for 1 h at room temperature, with gentle rocking. Following primary antibody incubation, membranes were washed 3 × 5 min in 5% skim milk in PBS-T at room temperature with gentle agitation. Membranes were probed with goat anti-rabbit horseradish peroxidase (HRP) secondary antibody diluted in Signal Boost secondary antibody diluent at 1:10,000 (40 ng/ml), or goat anti-mouse HRP secondary antibody diluted in 5% skim milk in PBS-T at 1:10,000 (40 ng/ml), for 1 h at room temperature, with gentle rocking. Membranes were washed 3 × 5 min in 5% skim milk in PBS-T at room temperature with gentle agitation, and proteins were visualized using enhanced chemiluminescence (ECL) on a LAS-4000 luminescence image analyser (Fujifilm). Exposure times are indicated in the figure legends for each blot.
HEK293 cell lysates were prepared as for immunoblotting, with the exclusion of DTT in the extraction buffer (EB–DTT). Protein concentration was determined from clarified lysates, and 800 µg total protein was transferred to fresh tubes and made up to 300 µl in EB–DTT. In total, 20 µl of diluted lysate was removed and mixed with 5× Laemmli sample buffer for immunoblot analysis. Immunoprecipitation was performed using the µMacs magnetic system (Miltenyi Biotec; see Table 3 for reagent details). Rabbit anti-SK2 antibodies (4 µg; ECM Biosciences 1:75 or Proteintech 1:52) or rabbit IgG isotype control antibody (4 µg), as well as 50 µl each of Protein A and G µBeads were added to the lysate, mixed gently and incubated on ice for 30 min. µMacs columns were placed onto a magnetic stand, equilibrated with 200 µl EB–DTT, and lysate/antibody/bead complexes were run through the columns. Columns were washed four times with 200 µl EB–DTT, and once with 100 µl low salt wash buffer, before the addition of 20 µl hot 1× Laemmli sample buffer for 5 min. Immunoprecipitates were then eluted with 50 µl hot 1× Laemmli sample buffer and collected in fresh tubes. Samples were boiled and 25 µl was analysed by SDS-PAGE and immunoblotting as described above.
Cell lines were seeded onto coverslips coated with poly-L-lysine (Sigma-Aldrich) in a 12-well plate (HEK293: 1.5 ×105 cells/well; HeLa: 7.5 ×104 cells/well; MEF: 3 ×104 cells/well). Cells were allowed to bed down overnight at 37°C in DMEM with 10% FBS. The following day (for HEK293 and HeLa cells), cells were treated with siRNA as described above, and incubated for 48 h. The following immunofluorescence staining protocol was then performed, with all steps carried out at room temperature (see Table 4 for reagent details). Cells were washed once in PBS, fixed in 4% paraformaldehyde for 10 min, washed three times in PBS-T, and permeabilized for 10 min in PBS-T. Cells were then blocked in 3% bovine serum albumin (BSA) in PBS-T for 30 min, and incubated for 1 h with anti-SK2 antibodies (4 µg/ml; ECM Biosciences 1:250 and Proteintech 1:172) diluted in 3% BSA/PBS-T. Cells were washed five times in PBS-T, and then incubated with goat anti-rabbit AlexaFluor 488 secondary antibody (1:500) for 1 h. After washing five times in PBS-T, cell nuclei were stained with DAPI (0.2 µg/ml) for 5 min. Cells were then washed twice in PBS, coverslips were partially dried and mounted onto slides using fluorescence mounting medium (Dako), and then left to set overnight. Fluorescence microscopy and imaging were performed using a Carl Zeiss LSM 700 confocal microscope, with Zen 2011 (Black Edition) version 8.1.5.484 software. All microscope settings, including gains, were kept constant for each cell line, allowing direct comparison between antibodies.
Various controls were used in these studies. Immunoprecipitations were performed with IgG isotype control antibody to control for any non-specific binding of proteins to the antibodies. Primary antibodies were also omitted from the immunofluorescence protocol to control for background fluorescence of the secondary antibody alone. siRNA-mediated SK2 knockdown as well as Sphk2-/- MEFs were utilized to verify the specificity of the SK2 antibodies to their target.
Both SK2 antibodies examined in the present study are reported by their respective manufacturers to be able to detect endogenous SK2 by IB. To determine the selectivity of the anti-SK2 antibodies, we performed IB analyses using two human cell lines (HEK293 and HeLa) that had been treated with either scrambled control or SK2-directed siRNA, as well as WT and Sphk2-/- MEFs. The Proteintech anti-SK2 antibody detected a single prominent band at the correct molecular weight for SK2 (~65 kDa), which was decreased or absent in the knockdown and knockout lines (Figure 1A; Dataset 118). Some faint non-specific bands were also detected in both the WT and Sphk2-/- MEF lysates by this antibody, which were not observed in the human cell lines. The ECM Biosciences anti-SK2 antibody did not appear to be very sensitive towards SK2, as no band was detected at the expected size in the HeLa lysates, and only very faint bands were present in the HEK293 and MEF lysates that were reduced or absent in the knockdown or knockout lines (Figure 1B). Furthermore, numerous prominent non-specific bands were present in all lysates, particularly in the MEF lines, indicating a lack of selectivity of this antibody towards SK2. Therefore, the Proteintech anti-SK2 antibody appears to be superior for use in IB, demonstrating both selectivity and sensitivity in the detection of endogenous SK2, particularly in the human cell lines tested.
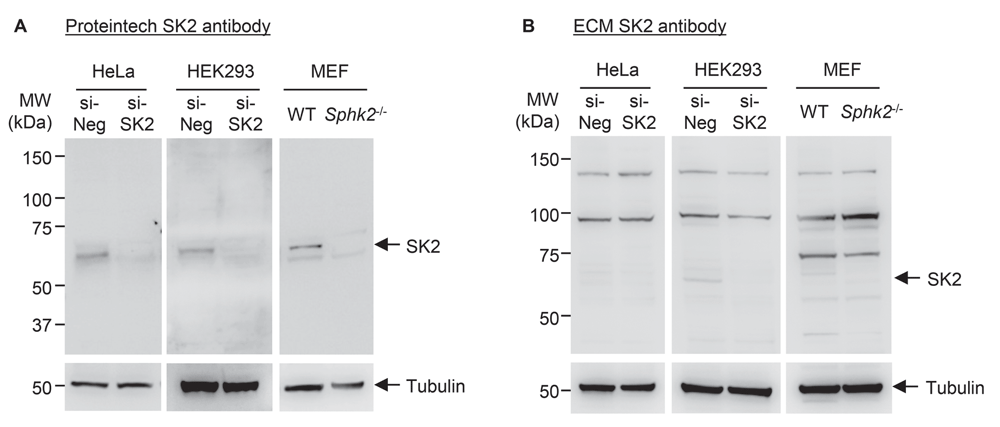
Immunoblot analyses of lysates from HEK293 and HeLa cells treated with scrambled control siRNA (si-Neg) or SK2 siRNA (si-SK2), and lysates from wildtype (WT) or Sphk2-/- MEFs. An equal amount (40 µg) of total protein from each sample was run in duplicate. After transferring to nitrocellulose and blocking, the membrane was separated and duplicate samples were probed with either (A) Proteintech rabbit anti-SK2 antibody or (B) ECM Biosciences rabbit anti-SK2 antibody. SK2 membranes were imaged using a 4 min exposure. The expected band size for SK2 is ∼65 kDa. Membranes were re-probed with mouse anti-α-tubulin antibody as a loading control (2 min exposure), which was detected at 55 kDa as expected. Consistent results were observed from 2-3 (HEK293 and MEF) or 3-4 (HeLa) independent experiments for each antibody.
We also examined whether either of the commercial anti-SK2 antibodies could immunoprecipitate SK2 from cell lysates. The Proteintech anti-SK2 antibody is suggested by the manufacturer to be useful for IP, whereas to our knowledge the ECM Biosciences anti-SK2 antibody has not been previously tested for use in this application. Initially, using lysates from HEK293 cells, we found that the Proteintech anti-SK2 antibody was sometimes able to IP a band at the correct size for SK2 (Figure 2A); however this was not consistent with each experimental repeat and other proteins were also immunoprecipitated to a varying extent by this antibody that were not present in the IgG isotype control (Dataset 219).

SK2 was immunoprecipitated from HEK293 cell lysate using either (A) Proteintech rabbit anti-SK2 antibody or (B) ECM Biosciences rabbit anti-SK2 antibody. Normal rabbit IgG antibody was used as an isotype control. Immunoprecipitates (and 40 µg lysate input) were subjected to immunoblot analyses and probed with (A) Proteintech rabbit anti-SK2 antibody or (B) ECM Biosciences rabbit anti-SK2 antibody. Membranes were imaged using a 4 min exposure. Images are representative of three independent experiments for each antibody. (C) SK2 was immunoprecipitated from HEK293 cell lysates (of equal protein) that had been treated with scrambled control siRNA (si-Neg) or SK2 siRNA (si-SK2), using ECM Biosciences rabbit anti-SK2 antibody. Immunoprecipitates were subjected to immunoblot analyses and probed with ECM Biosciences rabbit anti-SK2 antibody. Membrane was imaged using a 4 min exposure. Image is representative of three independent experiments. IgG h/c = IgG heavy chain.
Conversely, the ECM Biosciences anti-SK2 antibody was able to consistently and cleanly IP a protein of the same size as SK2 from cell lysates, with almost no non-specific bands observed (Figure 2B; Dataset 219). The protein immunoprecipitated by the ECM Biosciences antibody was considerably enriched from the cell lysate and was strongly detectable by this antibody, which was unable to detect SK2 in the lysate input sample, consistent with Figure 1B. To determine if this band was in fact SK2, the ECM Biosciences anti-SK2 antibody was then used to immunoprecipitate SK2 from HEK293 lysates treated with either scrambled control or SK2-directed siRNA. SK2 knockdown consistently resulted in reduced intensity of the band enriched by this antibody (Figure 2C; Dataset 219), confirming that the ECM Biosciences anti-SK2 antibody can selectively IP endogenous SK2.
Finally, we examined whether these commercially available SK2 antibodies could selectively detect SK2 by IF. Neither antibody has been reported to be tested for use in IF by their respective manufacturers; however, the Proteintech SK2 antibody is recommended for immunohistochemistry. Using IF staining methods routinely performed in our laboratory, we compared the two anti-SK2 antibodies using HeLa, HEK293 and MEF cell lines. The Proteintech anti-SK2 antibody produced minimal staining in all cell lines tested (Figure 3A–C), and consequently there was no observable differences between the control cells and those with SK2 knockdown (in the human cell lines) or SK2 knockout (in the Sphk2-/- MEF line).
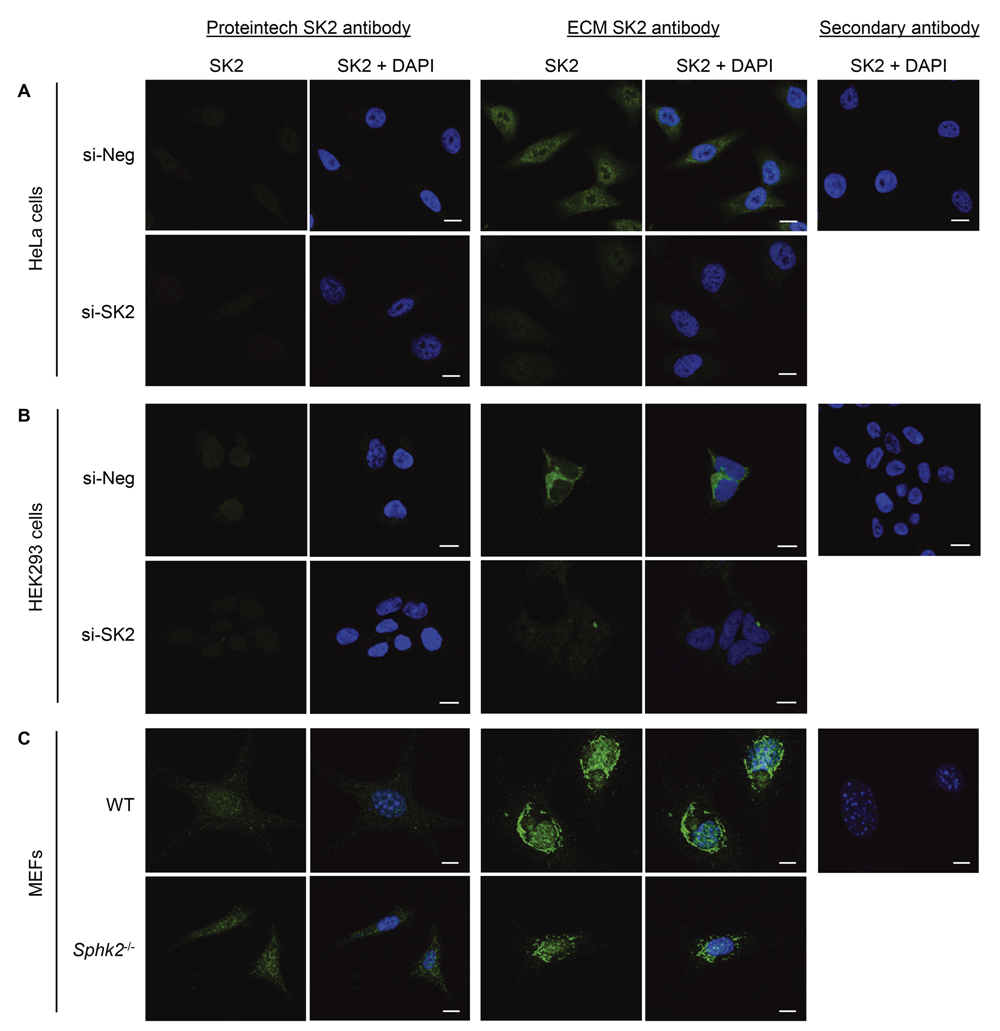
(A) HeLa or (B) HEK293 cells were treated with scrambled control siRNA (si-Neg) or SK2 siRNA (si-SK2), and endogenous SK2 (green) was visualised by immunofluorescence staining and confocal microscopy, using Proteintech rabbit anti-SK2 antibody or ECM Biosciences rabbit anti-SK2 antibody. (C) Wildtype (WT) or Sphk2-/- MEFs were seeded, and endogenous SK2 (green) was visualised by immunofluorescence staining and confocal microscopy, using Proteintech rabbit anti-SK2 antibody or ECM Biosciences rabbit anti-SK2 antibody. Nuclei were stained with DAPI (blue). For each cell line, background staining was examined by staining cells (si-Neg or WT cells) with secondary antibody and DAPI only, and collecting images using both 488nm and 405nm lasers (SK2 + DAPI). Images were taken at 40× magnification; scale bars = 10 µm. Images shown are representative of more than 100 cells from each experiment, and these results were consistent over three independent experiments for each cell line.
The ECM Biosciences anti-SK2 antibody did result in consistently observable staining in HeLa and HEK293 cells, which was substantially reduced upon knockdown of SK2 (Figure 3A and B; Dataset 320). Hence, in these cells the ECM Biosciences antibody was able to selectively detect SK2 by IF. Interestingly, in HeLa cells SK2 detected by the ECM Biosciences antibody was predominantly nuclear with some peri-nuclear/cytoplasmic localization, whereas in HEK293 cells SK2 was cytoplasmic and nuclear-exclusion, which is consistent with previous reports for these cell lines9. However, the ECM Biosciences anti-SK2 antibody produced very strong peri-nuclear staining/puncta in both the WT and Sphk2-/- MEF lines (Figure 3C), suggesting that this staining was not specific for SK2 and represents non-specific binding to other proteins in this cell type. Increased non-specific binding of both SK2 antibodies to other proteins in the MEF lines was also observed when used for IB, so this cell type may not be suitable for use with these antibodies. It will remain to be determined if the same level of non-specificity is also observed in other mouse cell lines and tissues.
Commercially available antibodies raised against the SKs can be notorious, in our experience, for not being very sensitive or selective. This is accentuated by the apparent low abundance of SK proteins in most cells, which can be in some cases hundreds of fold lower than when overexpressed4,21. A number of groups have generated their own SK-specific antibodies; however, many published studies have reported the use of different commercial SK2 antibodies, sometimes without proper controls or validation of selectivity. Hence, we have compared two commercially available SK2 antibodies and evaluated their selectivity towards SK2 in multiple applications using siRNA-mediated SK2 knockdown or Sphk2-/- MEF lines.
We found that the SK2 antibody from Proteintech was able to consistently detect a prominent band at the correct molecular weight by IB, and this band was confirmed to be SK2 by knockdown and knockout analyses, confirming the specificity of this antibody. The Proteintech antibody also resulted in virtually no non-specific detection of any other proteins in the HEK293 and HeLa lysates, but some additional faint bands were present in the MEF lines. This antibody has been tested by IB on various mouse tissue lysates by the manufacturer and many of these also gave rise to non-specific bands, so this will need to be considered and further validation may be required if this antibody is intended for use with mouse cells or tissues. Occasionally more than one band was detected in the human cell lines by the Proteintech SK2 antibody, but these bands also seemed to be reduced by SK2 knockdown. There are two characterized human SK2 isoforms22, so these bands may represent different SK2 variants and/or post-translationally modified forms of SK2. Notably, although a previous report suggested that the mRNA of the 69 kDa SK2b (SK2-L) isoform is the most abundant in many human cell lines, including HeLa cells6, our studies clearly suggest that the SK2a (SK2-S) isoform is the predominant form of SK2 protein in HeLa and HEK293 cells.
In contrast, the present results revealed that the sensitivity of the ECM Biosciences antibody towards SK2 by IB was poor, with a faint band detected only in the HEK293 and MEF lines that was not present in the knockdown/knockout lysates. Furthermore, the ECM Biosciences SK2 antibody produced many intense non-specific bands in all cell lines tested, demonstrating poor selectivity. This antibody has been used for IB analyses in multiple publications12–15, suggesting that it may be more suitable with other cell/tissue systems or conditions not tested here. However, in agreement with our findings, the IB analysis performed by the manufacturer also showed various prominent non-specific bands in HeLa lysates. Therefore, at least in our hands, the ECM Biosciences SK2 antibody was not ideal for this application.
However, the ECM Biosciences anti-SK2 antibody was superior for the IP of endogenous SK2, as it was able to cleanly and substantially enrich the protein from lysates and was confirmed by SK2-specific knockdown to be selective for SK2 in this application. This antibody will therefore be a useful tool to study SK2 function and regulation, as it can be applied to other applications requiring IP, such as chromatin-IP (ChIP) and rapid immunoprecipitation mass spectrometry of endogenous protein (RIME). In the present study, the Proteintech anti-SK2 antibody was inconsistent in its ability to IP protein at the correct size for SK2, and other bands of equal intensity were sometimes present.
Similarly, we found that the ECM Biosciences anti-SK2 antibody was able to selectively detect endogenous SK2 by IF staining in two human cell lines, HeLa and HEK293 cells. Furthermore, the observed localization of SK2 in these two cells lines was consistent with previous reports9. The selectivity of this antibody was validated by knockdown of SK2 in these cell lines, where most of the staining was reduced. A very small level of staining was still visible after SK2 siRNA treatment, possibly owing to the inherently incomplete nature of siRNA-mediated knockdown. However, we were unable to corroborate these data with SK2 knockout using the MEF lines, as considerable non-specific staining was present in this cell type, as was found for IB. Using identical methods, there was minimal staining observed using the Proteintech anti-SK2 antibody for IF, and therefore the selectivity of this antibody towards SK2 in this application could not be properly examined.
During this study, methods routinely used in our laboratory were employed, and where applicable, recommendations from the manufacturers for antibody dilutions and concentrations were followed. It is possible that further optimization for these antibodies may allow them to perform better in the applications where they were deemed not optimal. However, as our main aim was to directly compare the performance of these two antibodies, and given at least one of the antibodies performed well for each application using our standard methods, further optimization was not performed.
Overall, based on the data from this study we would recommend the use of the Proteintech SK2 antibody for IB, as it demonstrated selectivity and sensitivity towards endogenous SK2 in the human cell lines tested. Furthermore, we recommend the ECM Biosciences SK2 antibody for IP of endogenous SK2 and for visualizing SK2 by IF methods. Furthermore, both antibodies detected non-specific proteins by IB and IF in the mouse fibroblasts used, and hence further validation will be required to determine if this is the case for other mouse cells or tissues.
Dataset 1: Raw images of all experimental replicates for Figure 1, immunoblotting experiments. This dataset includes uncropped blots for all experimental replicates that are represented in Figure 1. Treatments and immunoblot methods were performed as outlined in Figure 1. Blots were probed with Proteintech rabbit polyclonal anti-SK2 antibody (A–D) or ECM Biosciences rabbit polyclonal anti-SK2 antibody (E–H). Anti-α-tubulin antibody was used as a loading control. O/E SK2 = lysate from cells overexpressing SK2, used as a positive control to validate the correct size of SK2. Asterisks denote other protein bands that were probed using other antibodies not relevant to this study, prior to anti-α-tubulin.
DOI, 10.5256/f1000research.10336.d14541618
Dataset 2: Raw images of all experimental replicates for Figure 2, immunoprecipitation experiments. This dataset includes uncropped blots for all experimental replicates that are represented in Figure 2. SK2 immunoprecipitation from HEK293 cell lysate, and subsequent immunoblotting, were performed using either (A–C) Proteintech rabbit anti-SK2 antibody or (D–F) ECM Biosciences rabbit anti-SK2 antibody. (G–I) SK2 immunoprecipitation from HEK293 cell lysates (of equal protein) treated with scrambled control siRNA (si-Neg) or SK2 siRNA (si-SK2), and subsequent immunoblotting, were performed using ECM Biosciences rabbit anti-SK2 antibody.
DOI, 10.5256/f1000research.10336.d14541719
Dataset 3: Raw images of additional experimental replicates for Figure 3, immunofluorescence experiments. This dataset includes additional images from experimental replicates that demonstrate reproducibility of the images presented in Figure 3. Treatments and immunofluorescence staining methods were performed as outlined in Figure 3. Images were taken at 40× magnification; scale bars = 10 µm.
SP conceived the study. HN designed and carried out the experiments, and prepared the first draft of the manuscript. Both authors were involved in the revision of the draft manuscript and have agreed to the final content.
This work was funded by an Australian Postgraduate Award, Royal Adelaide Hospital Dawes Scholarship and the University of South Australia (HN), and a National Health and Medical Research Council of Australia Project Grant (#626936) and Senior Research Fellowship (#1042589), and the Fay Fuller Foundation (SP).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank Dr Briony Gliddon for generating the primary mouse embryonic fibroblasts used in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 23 Mar 17 |
||
|
Version 1 06 Dec 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)