Introduction
Respiratory syncytial virus (RSV) is the most common trigger of bronchiolitis and viral pneumonia, especially in infants, and there are links between severe RSV disease and later development of asthma and wheeze1–3. There are at present no effective RSV anti-virals or RSV vaccines in the clinic; therefore, infection with the virus remains a clinical problem worldwide, and avoiding the development of severe lower respiratory tract infection constitutes an unmet need. There are many known risk factors for severe RSV disease such as pre-term birth, lung underdevelopment, and congenital heart disease4,5. However, previously healthy babies lacking any of the above risk factors are also admitted to hospital with severe lower respiratory tract RSV infection1–3,6. Possible parameters determining the severity of disease include genetic susceptibility of the host, presence of co-infections with other pathogens, viral genotype, and viral load (Figure 14–6). However, other reasons relate to how immune responses to the virus are induced and regulated, an area about which we still know very little (Figure 1). Innate immune responses occur immediately upon infection and are important for the early containment of pathogens before adaptive immune responses (antibodies and T cells) can be mobilised. They also direct subsequent adaptive immune responses and dictate how strongly the host responds to the invading pathogen. Innate immune responses are difficult to investigate during natural RSV infection, especially in children, as they have generally waned by the time of hospital visit/admission. However, experimental models of RSV infection can be used to begin to understand how innate immunity to the virus is elicited and impacts disease progression. In this commentary, recent advances in understanding RSV infection are summarised, with a focus on new findings in the area of innate immunity to the virus.
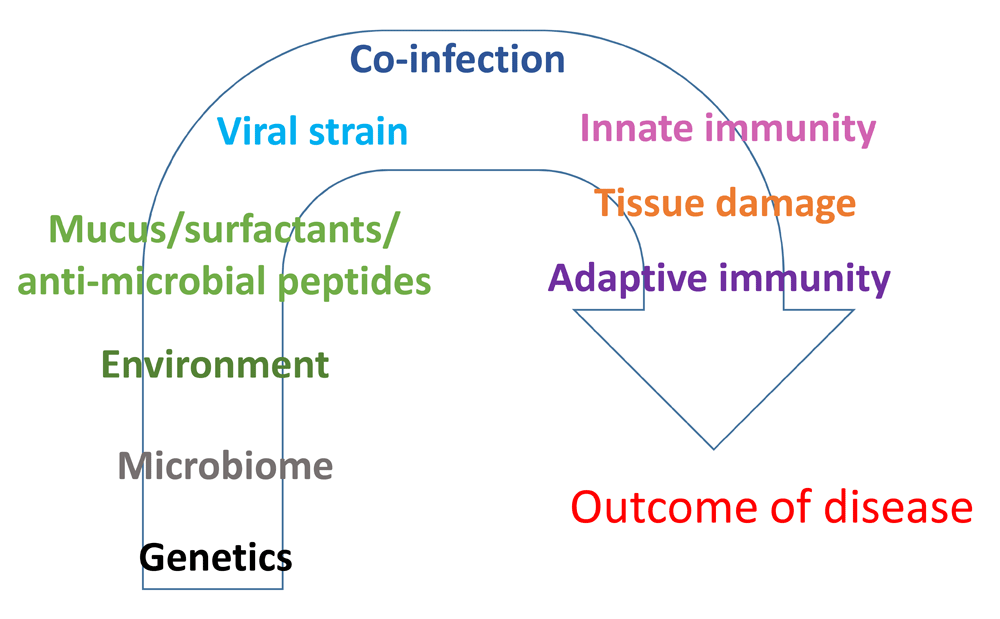
Figure 1. Possible determinants of severity of disease during respiratory syncytial virus (RSV) infection.
Many host, environmental, or viral factors can determine the outcome and severity of RSV disease. Most likely an interplay of several factors will determine why some patients develop severe disease.
RSV infection
RSV is a negative sense, single-stranded RNA virus of the Pneumoviridae family (previously classified in the Paramyxoviridae family7,8). It was first described in chimpanzees in 19559 and shortly thereafter detected in children with respiratory illness10. RSV is estimated to cause 34 million episodes of lower respiratory tract infections leading to 3.4 million hospitalisations and up to 199,000 deaths per year in children younger than 5 years of age11. Hospitalisation is most common in infants between 2 and 6 months of age6.
RSV infects the respiratory tract by initially binding to molecules on the apical surface of epithelial cells or by non-specific uptake via macropinocytosis7,12. Which receptors are involved in binding the virus and facilitating infection is not fully elucidated, but several cell surface molecules have been implicated in the process. For example, glycosaminoglycans expressed on cell surfaces can bind to the envelope glycoproteins of RSV, namely the G and F proteins. RSV G is important for viral attachment to the host cells, while RSV F is involved in the fusion of the viral envelope with either the cell plasma membrane or the delimiting membrane of macropinosomes7,12. RSV F protein expressed on the surface of neighbouring infected cells also causes their fusion to form syncytia, a characteristic feature of the infection that lends the virus its name7. RSV F protein also binds the cellular protein nucleolin and this increases infection13. In addition, CX3CR1 (the fractalkine receptor) was recently shown to be expressed on ciliated epithelial cells and can bind to RSV G14–16, since RSV G contains a CX3C motif17,18. Notably, mice lacking CX3CR1 are less susceptible to RSV infection15, underscoring the importance of this interaction in viral entry. Following attachment, and fusion, RSV enters the cytoplasm and the replication cycle ensues. Progeny viruses eventually assemble and bud off the plasma membrane after the formation of long protruding structures called filaments7. Released viral particles then infect neighbouring cells and propagate the infectious process.
Whether lower airway disease is caused by uncontrolled virus infection resulting in syncytial cell death and epithelial barrier breakdown or whether it is due to tissue damage caused by a dysregulated immune response (immunopathology) is not fully understood. Importantly, the two are not independent variables. A high viral load has been associated with high release of pro-inflammatory immune mediators and more severe symptoms19–23. Thus, it is possible that the development of severe disease is due to an early lack of control of the virus, which leads to epithelial cell damage and a high release of pro-inflammatory mediators that recruit and activate leukocytes in the lung and induce an excessive immune response that results in immunopathology20,24–26. The risk groups for severe RSV disease are the young (less than one year of age) and the old (more than 65 years of age)27,28. Infants have an immature immune system, which renders them less able to mount an efficient anti-viral response28,29. In addition, it is likely that structural features including small airway calibre may make infants more prone to critical airway narrowing and resultant hypoxia in the face of lung inflammation28. The elderly have a senescing immune system and are therefore less able to induce appropriate responses to invading pathogens30,31. It is possible that the innate immune response in these two at-risk groups is suboptimal, which results in an unbalanced immune response and inefficient balance between viral control and immunopathology.
Innate immune responses to RSV
RSV research has long been focused on adaptive immunity. Recently, the importance of innate immunity has been highlighted, especially from studies using animal models. There are many factors that can influence the development and severity of disease (Figure 1). The first lines of defence are mucus32, anti-microbial peptides33, and surfactants34,35. The local lung microbiota can also most likely influence RSV infection rate and the immune response to the virus, but this is an emerging concept for which, at present, there are limited supporting data. However, it has recently been suggested that the nasopharyngeal microbiota in young children can influence the spread of the infection to the lower airways and modulate the host immune response to RSV infection36,37. It remains to be elucidated if the composition of the microbiota is changed by the infection or if a specific composition of microbiota is determining the degree and spread of infection.
The next layer of defence the virus has to confront is the resident cells of the respiratory tract, mainly epithelial cells, alveolar macrophages (AMs), dendritic cells (DCs), and innate lymphoid cells. Many cells express pattern recognition receptors (PRRs) that can bind to pathogen-associated molecular patterns (PAMPs) and signal to initiate the production of pro-inflammatory cytokines and chemokines that serve to orchestrate anti-viral immunity. Some of these have potent anti-viral effects themselves, such as the type I interferons (IFN-α/β)38,39. These cytokines are transiently produced and bind to the type I IFN receptor (IFNAR) expressed on all nucleated cells to signal to induce the expression of a large number of proteins that help restrict viral replication. More recently, it has become apparent that type I IFNs also play a key role in inducing cytokines and chemokines that promote the recruitment and activation of immune cells24,25,38. As a counter-strategy, many viruses have evolved proteins that hinder type I IFN production or block IFNAR signalling. RSV has two non-structural proteins, NS1 and NS2, that inhibit type I IFN production and signalling in infected cells40 as well as interfere with epithelial cell sloughing41. In addition, RSV N protein has been suggested to also be able to inhibit IFN-β induction42. Furthermore, viruses, including RSV, can subvert the cell-intrinsic anti-viral responses by manipulating microRNA generation and/or function43,44.
Genetic analyses of infants show an association of single nucleotide polymorphisms (SNPs) in genes encoding type I IFNs or proteins involved in IFNAR signalling with severe RSV disease45,46. Also, a deficiency in type I IFN production by cells from infants47 and from neonatal mice48,49 has been shown. In contrast, some studies show a higher level of IFN-α in nasopharyngeal wash in more severely sick infants compared to controls50. It is possible that the type I IFN response is not detectable by the time children are admitted to hospital with lower respiratory tract RSV infection, since this is likely to happen several days after the initial infection, at which time the production of type I IFNs is declining and these cytokines are difficult to detect.
Cytosolic PRRs of the RIG-I-like receptor (RLR) family that signal via mitochondrial anti-viral signalling protein (MAVS) are crucial for the production of type I IFNs and other pro-inflammatory cytokines during RSV infection24,26,51. UV-inactivated RSV does not elicit type I IFN responses24,52, but defective RSV genomes can in both mice and humans53. Interestingly, the major sources of type I IFNs in the lower airways of mice during experimental RSV infection are AMs24, and they use cytosolic MAVS-coupled sensors to induce this production24,52. However, these cells are not productively infected by RSV52.
Several Toll-like receptors (TLRs), such as TLR2, 3, 4, and 7, are also implicated in the recognition of RSV54,55. For example, RSV F can bind TLR456 and SNPs in the TLR4 gene correlate with severe RSV disease57–59. However, mouse models show variable dependency on TLR4 for the development of disease56,60,61. TLR4 is best known as a receptor for lipopolysaccharide (LPS), a bacterial product, and a recent study showed that an intersection of TLR4 genotype with LPS content in the home environment determines the severity of RSV disease62. This indicates that the interplay between genetics and the environment, including the microbiota and co-infections, will be part of the severity of disease caused by RSV (Figure 1). Interestingly, even mice genetically lacking the ability to signal via all TLRs and RLRs are able to control RSV infection and mount T cell responses to the virus63. This suggests that additional mechanisms for detecting RSV infection exist that can compensate for the lack of PRR signalling. Like PAMPs, damage-associated molecular patterns (DAMPs) released by dead cells can trigger immunity64. One part of RSV disease manifestation is small airway obstruction caused by a mix of mucus, infiltrating cells, and dead or dying epithelial and inflammatory cells1,6. Many DAMPs will therefore at this stage be expected to be present freely in the lungs and might contribute to the initiation of immune responses to the virus. Recently, RSV infection was shown to trigger the release of DAMPs such as high mobility group box 1 (HMGB1)65 and S100A966.
PAMP or DAMP recognition often results in the production of pro-inflammatory cytokines and chemokines, many of which, including TNF, IL-6, and CCL2, have been associated with severe lower respiratory tract infection and recurrent wheeze6,67. For example, recently the epithelial-derived cytokine IL-33 was shown to contribute to disease severity in neonates and was also found in nasal aspirates from infants after RSV infection68. Locally produced cytokines are important for lung cell proliferation, activation, and differentiation, and chemokines are important for orchestrating immune cell infiltration into the lungs. One of the first cell types to be recruited after lung infections is neutrophils. They infiltrate the lung in vast numbers during RSV infection25,69–71 and have multiple functions such as phagocytosis, production of reactive oxygen species, and secretion of proteolytic enzymes72. Activated neutrophils can also form neutrophil extracellular traps (NETs), networks of DNA and microbicidal proteins, that can capture RSV73,74. However, whether neutrophils are beneficial for viral control or, rather, become a cause of lung injury/occlusion during RSV infection needs further investigation72. The next cell type to arrive in the lung is the monocytes. Type I IFNs are instrumental for recruiting inflammatory monocytes by inducing the production of monocyte chemoattractants such as CCL2. The monocytes are important for controlling the virus and have recently been shown to contribute to viral clearance/control of RSV in the lower airways of mice24. Interestingly, children with bronchiolitis display high levels of CCL2 in nasopharyngeal wash50 and in bronchoalveolar lavage (BAL) fluid75,76. In the mouse model of RSV infection, lung monocytes are anti-viral and contribute to host protection24, while in a model of influenza virus infection monocytes cause pathology77,78. It is interesting to speculate that increased/uncontrolled levels of type I IFNs could recruit high numbers of monocytes that are initially important for viral control but, in excess, become detrimental and contribute to lung immunopathology.
Natural killer (NK) cells and, subsequently, T cells infiltrate the lungs following neutrophils and monocytes. Both of these cell types have an anti-viral effect during RSV infection79–81. Each infiltrating cell type has a role in anti-viral defence, and its recruitment is well orchestrated in order to clear the virus while limiting tissue damage, a particularly important issue in delicate tissues, such as the lung, which needs to preserve gas exchange function irrespective of ongoing infectious challenge. If lung recruitment of immune cell types is dysregulated, the balance of viral control versus tissue damage is lost, and pathology and severe disease can ensue. Thus, innate immune responses to RSV are crucial in executing the initial control of the virus and in directing a balanced immune response.
Adaptive immune responses to RSV
DCs are key in the crosstalk between innate and adaptive immunity82. They are activated by PRR signalling and pro-inflammatory mediators in the lung to increase their antigen processing and presentation capability and migrate to regional lymph nodes where they convey viral antigens to naïve T and B cells83. It is interesting to note that there are fewer DCs in the lungs and lymph nodes of infants and they are also less functional after RSV infection compared to those from adult lungs49,84,85. Also, fewer and less functional plasmacytoid DCs are found in young children47,86. Flt3 ligand subcutaneous administration to neonatal mice mobilises more lung DCs and results in less lung inflammation after re-infection with RSV49. Altogether, these data suggest that a correct DC response is vital for a balanced immune response during RSV infection.
RSV-specific B and T cells are activated in the lymph nodes and proliferate, migrate, and start executing their respective functions a few days after the start of the RSV infection. The antibodies secreted by RSV-specific B cells are important to prevent viral spread and reinfection. Interestingly, RSV-specific antibodies have very short half-life and serum titres, and the number of IgA+ memory B cells decreases with time87–89. This is unlike other respiratory infections, such as with influenza virus, in which antibodies persist and confer lifelong protection against the original infecting strain. However, nasal anti-RSV IgA levels correlate with protection from experimental infection in adults89, while levels of nasal (maternally derived) anti-RSV IgG correlate with lower viral load in infants90. Furthermore, anti-RSV antibodies have been found in amniotic fluid91 and could potentially be protective to the lungs of newborns. Altogether, these data indicate a possible beneficial role for antibodies during RSV infection but raise the conundrum of why protective antibody-dependent responses fail to establish memory in adults, permitting re-infection with the same RSV strain.
T cells arrive in the lungs from the lymph nodes a few days after the start of RSV infection. They are important for viral clearance during primary infection92, and protective responses to RSV infection are characterised by a T helper 1 (Th1)-dominated response with T cells that produce IFN-γ (CD4+ and CD8+ T cells) and kill infected cells with perforin and granzyme B (CD8+ T cells)93. In contrast, if the T cell responses are skewed towards a Th2 type, they can contribute to immunopathology62,93–95. Although, more recently, some of these observations have also been attributed to Th17 induction96.
After a first infection with RSV, memory T cells are generated and can be mobilised when the host re-encounters RSV at a later stage. There are different types of memory T cells: central memory, effector memory, and tissue-resident memory (TRM) T cells. RSV-specific TRM cells localise to the lungs and can exert an innate-like function when reinfection occurs97,98. TRM cells have been found in mouse99 and human100 lungs after RSV infection has cleared. Even though memory T cell responses develop during RSV infection, their longevity has been debated, as re-infections with the same virus strain occur throughout life. Also, the type of memory T cells (Th1, Th2, or Th17) is important for the outcome of the reinfection, with an increased Th2 or Th17 response correlating with enhanced disease93. Increasing evidence suggests that TRM cells are crucial for protection from re-infection with various pathogens and therefore an important consideration for vaccine development (see below). Future research will inform a more precise role of TRM cells during RSV infection.
A strong or dysregulated T cell response to RSV will be detrimental to lung tissue integrity. CD8+ and CD4+ T cells start to upregulate IL-10 production during RSV infection101–104 most likely in order to dampen the ongoing immune response, as IL-10 can have anti-inflammatory effects105. Also, T regulatory cells (Tregs) are important for keeping the T-cell-driven inflammation in check, especially the Th17 and Th2 types, during RSV infection96,106–112. Thus, adaptive immune responses to RSV are important for the final viral clearance and for a rapid memory response in case of a re-infection, but this response also has to be under tight control in order to limit immunopathology.
Potential vaccines
To date, the treatment of RSV disease is mainly supportive and no specific anti-virals or vaccines are currently licenced1,113. It has proven difficult to achieve viral control without causing immunopathology. In the 1960s, a trial using formalin-inactivated RSV failed to induce protection in vaccinated children but instead enhanced disease after natural infection with RSV114. An issue for vaccine development is the lack of correlates of protection coupled to the difficulties of lung sampling, which is crucial as protective immune responses to RSV will not necessarily be evident in the blood99,100. Despite these issues, several vaccines (subunit, live-attenuated, and vector vaccines) and routes of vaccination are currently being developed and tested113,115,116. In addition, the adjuvants used for triggering the innate immune responses during vaccination are an important avenue of ongoing research to find an effective vaccine. Passive immunisation has also proven useful, and Palivizumab, a monoclonal antibody against RSV F protein, is given to high-risk infants to prevent infection and the development of severe disease117. Recently, an anti-RSV G monoclonal antibody was shown to be more effective than anti-F in preventing RSV disease in animal models118, but this has yet to be tested in humans. Finally, maternal vaccination against RSV is an interesting future avenue for generating protection in the first month of life in newborns via placental or breast milk transfer of maternal RSV-specific antibodies to the infant119.
Conclusions
The understanding of the causes and mechanisms of RSV disease has increased tremendously over the last few years. However, many unknown factors are yet to be discovered. The influence of the microbiota and environment on the severity of disease as well as the understanding of the specific roles of individual lung and immune cells during the infection are still waiting to be unveiled. The knowledge of basic mechanisms will be instrumental for the understanding of disease progression, outcome, and severity and will more efficiently guide vaccine and therapy development in the future.
Abbreviations
AMs, alveolar macrophages; BAL, bronchoalveolar lavage; DAMPs, damage-associated molecular patterns; DCs, dendritic cells; IFNAR, type I interferon receptor; IFNs, interferons; LPS, lipopolysaccharide; MAVS, mitochondrial anti-viral signalling protein; PAMPs, pathogen-associated molecular patterns; PRRs, pattern recognition receptors; RLRs, RIG-I-like receptors; RSV, respiratory syncytial virus; SNP, single nucleotide polymorphism; Th, T helper; TLRs, Toll-like receptors; TRM cells, tissue-resident memory T cells.
Competing interests
The author declares that she has no competing financial interests.
Grant information
I want to thank the Medical Research Council (Grant G0800311), the Rosetrees Trust (M370), and the National Heart and Lung Institute Foundation (registered charity number 1048073) for research support.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
I wish to thank Caetano Reis e Sousa and Spyridon Makris for critical reading of the manuscript.
Faculty Opinions recommendedReferences
- 1.
Meissner HC:
Viral Bronchiolitis in Children.
N Engl J Med.
2016; 374(1): 62–72. PubMed Abstract
| Publisher Full Text
- 2.
Blanken MO, Rovers MM, Molenaar JM, et al.:
Respiratory syncytial virus and recurrent wheeze in healthy preterm infants.
N Engl J Med.
2013; 368(19): 1791–9. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 3.
Sigurs N, Aljassim F, Kjellman B, et al.:
Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life.
Thorax.
2010; 65(12): 1045–52. PubMed Abstract
| Publisher Full Text
- 4.
Hervás D, Reina J, Yañez A, et al.:
Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis.
Eur J Clin Microbiol Infect Dis.
2012; 31(8): 1975–81. PubMed Abstract
| Publisher Full Text
- 5.
Murray J, Bottle A, Sharland M, et al.:
Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study.
PLoS One.
2014; 9(2): e89186. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 6.
Borchers AT, Chang C, Gershwin ME, et al.:
Respiratory syncytial virus--a comprehensive review.
Clin Rev Allergy Immunol.
2013; 45(3): 331–79. PubMed Abstract
| Publisher Full Text
- 7.
Collins PL, Karron RA:
Fields Virology. 6 ed. Knipe DM, Howley PM, editors. Lippincott Williams & Wilkins; 2013; 1: 38.
- 8.
Afonso CL, Amarasinghe GK, Bányai K, et al.:
Taxonomy of the order Mononegavirales: update 2016.
Arch Virol.
2016; 161(8): 2351–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Blount RE Jr, Morris JA, Savage RE:
Recovery of cytopathogenic agent from chimpanzees with coryza.
Proc Soc Exp Biol Med.
1956; 92(3): 544–9. PubMed Abstract
- 10.
Chanock R, Roizman B, Myers R:
Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization.
Am J Hyg.
1957; 66(3): 281–90. PubMed Abstract
- 11.
Nair H, Nokes DJ, Gessner BD, et al.:
Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis.
Lancet.
2010; 375(9725): 1545–55. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 12.
Krzyzaniak MA, Zumstein MT, Gerez JA, et al.:
Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein.
PLoS Pathog.
2013; 9(4): e1003309. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Tayyari F, Marchant D, Moraes TJ, et al.:
Identification of nucleolin as a cellular receptor for human respiratory syncytial virus.
Nat Med.
2011; 17(9): 1132–5. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 14.
Chirkova T, Lin S, Oomens AG, et al.:
CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells.
J Gen Virol.
2015; 96(9): 2543–56. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 15.
Johnson SM, McNally BA, Ioannidis I, et al.:
Respiratory Syncytial Virus Uses CX3CR1 as a Receptor on Primary Human Airway Epithelial Cultures.
PLoS Pathog.
2015; 11(12): e1005318. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 16.
Jeong K, Piepenhagen PA, Kishko M, et al.:
CX3CR1 Is Expressed in Differentiated Human Ciliated Airway Cells and Co-Localizes with Respiratory Syncytial Virus on Cilia in a G Protein-Dependent Manner.
PLoS One.
2015; 10(6): e0130517. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 17.
Chirkova T, Boyoglu-Barnum S, Gaston KA, et al.:
Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses.
J Virol.
2013; 87(24): 13466–79. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Tripp RA, Jones LP, Haynes LM, et al.:
CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein.
Nat Immunol.
2001; 2(8): 732–8. PubMed Abstract
| Publisher Full Text
- 19.
DeVincenzo JP, Wilkinson T, Vaishnaw A, et al.:
Viral load drives disease in humans experimentally infected with respiratory syncytial virus.
Am J Respir Crit Care Med.
2010; 182(10): 1305–14. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 20.
El Saleeby CM, Bush AJ, Harrison LM, et al.:
Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children.
J Infect Dis.
2011; 204(7): 996–1002. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
DeVincenzo JP, El Saleeby CM, Bush AJ:
Respiratory syncytial virus load predicts disease severity in previously healthy infants.
J Infect Dis.
2005; 191(11): 1861–8. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 22.
Houben ML, Coenjaerts FE, Rossen JW, et al.:
Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community.
J Med Virol.
2010; 82(7): 1266–71. PubMed Abstract
| Publisher Full Text
- 23.
Scagnolari C, Midulla F, Selvaggi C, et al.:
Evaluation of viral load in infants hospitalized with bronchiolitis caused by respiratory syncytial virus.
Med Microbiol Immunol.
2012; 201(3): 311–7. PubMed Abstract
| Publisher Full Text
- 24.
Goritzka M, Makris S, Kausar F, et al.:
Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes.
J Exp Med.
2015; 212(5): 699–714. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Goritzka M, Durant LR, Pereira C, et al.:
Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection.
J Virol.
2014; 88(11): 6128–36. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Bhoj VG, Sun Q, Bhoj EJ, et al.:
MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus.
Proc Natl Acad Sci U S A.
2008; 105(37): 14046–51. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 27.
Falsey AR, McElhaney JE, Beran J, et al.:
Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness.
J Infect Dis.
2014; 209(12): 1873–81. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 28.
Ruckwardt TJ, Morabito KM, Graham BS:
Determinants of early life immune responses to RSV infection.
Curr Opin Virol.
2016; 16: 151–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Lambert L, Sagfors AM, Openshaw PJ, et al.:
Immunity to RSV in Early-Life.
Front Immunol.
2014; 5: 466. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Pinti M, Appay V, Campisi J, et al.:
Aging of the immune system: Focus on inflammation and vaccination.
Eur J Immunol.
2016; 46(10): 2286–301. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 31.
Meyer KC:
The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly.
Semin Respir Crit Care Med.
2010; 31(5): 561–74. PubMed Abstract
| Publisher Full Text
- 32.
Zanin M, Baviskar P, Webster R, et al.:
The Interaction between Respiratory Pathogens and Mucus.
Cell Host Microbe.
2016; 19(2): 159–68. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 33.
Currie SM, Gwyer Findlay E, McFarlane AJ, et al.:
Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans.
J Immunol.
2016; 196(6): 2699–710. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 34.
LeVine AM, Gwozdz J, Stark J, et al.:
Surfactant protein-A enhances respiratory syncytial virus clearance in vivo.
J Clin Invest.
1999; 103(7): 1015–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 35.
LeVine AM, Elliott J, Whitsett JA, et al.:
Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus.
Am J Respir Cell Mol Biol.
2004; 31(2): 193–9. PubMed Abstract
| Publisher Full Text
- 36.
de Steenhuijsen Piters WA, Heinonen S, Hasrat R, et al.:
Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection.
Am J Respir Crit Care Med.
2016; 194(9): 1104–15. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 37.
Teo SM, Mok D, Pham K, et al.:
The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development.
Cell Host Microbe.
2015; 17(5): 704–15. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 38.
Durbin RK, Kotenko SV, Durbin JE:
Interferon induction and function at the mucosal surface.
Immunol Rev.
2013; 255(1): 25–39. PubMed Abstract
| Publisher Full Text
- 39.
McNab F, Mayer-Barber K, Sher A, et al.:
Type I interferons in infectious disease.
Nat Rev Immunol.
2015; 15(2): 87–103. PubMed Abstract
| Publisher Full Text
- 40.
van Drunen Littel-van den Hurk S, Watkiss ER:
Pathogenesis of respiratory syncytial virus.
Curr Opin Virol.
2012; 2(3): 300–5. PubMed Abstract
| Publisher Full Text
- 41.
Liesman RM, Buchholz UJ, Luongo CL, et al.:
RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction.
J Clin Invest.
2014; 124(5): 2219–33. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Lifland AW, Jung J, Alonas E, et al.:
Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS.
J Virol.
2012; 86(15): 8245–58. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
tenOever BR:
RNA viruses and the host microRNA machinery.
Nat Rev Microbiol.
2013; 11(3): 169–80. PubMed Abstract
| Publisher Full Text
- 44.
Aguado LC, Schmid S, Sachs D, et al.:
microRNA Function Is Limited to Cytokine Control in the Acute Response to Virus Infection.
Cell Host Microbe.
2015; 18(6): 714–22. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 45.
Janssen R, Bont L, Siezen CL, et al.:
Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes.
J Infect Dis.
2007; 196(6): 826–34. PubMed Abstract
| Publisher Full Text
- 46.
Siezen CL, Bont L, Hodemaekers HM, et al.:
Genetic susceptibility to respiratory syncytial virus bronchiolitis in preterm children is associated with airway remodeling genes and innate immune genes.
Pediatr Infect Dis J.
2009; 28(4): 333–5. PubMed Abstract
| Publisher Full Text
- 47.
Marr N, Wang TI, Kam SH, et al.:
Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children.
J Immunol.
2014; 192(3): 948–57. PubMed Abstract
| Publisher Full Text
- 48.
Cormier SA, Shrestha B, Saravia J, et al.:
Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection.
J Virol.
2014; 88(16): 9350–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 49.
Remot A, Descamps D, Jouneau L, et al.:
Flt3 ligand improves the innate response to respiratory syncytial virus and limits lung disease upon RSV reexposure in neonate mice.
Eur J Immunol.
2016; 46(4): 874–84. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 50.
Tabarani CM, Bonville CA, Suryadevara M, et al.:
Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection.
Pediatr Infect Dis J.
2013; 32(12): e437–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 51.
Demoor T, Petersen BC, Morris S, et al.:
IPS-1 signaling has a nonredundant role in mediating antiviral responses and the clearance of respiratory syncytial virus.
J Immunol.
2012; 189(12): 5942–53. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 52.
Makris S, Bajorek M, Culley FJ, et al.:
Alveolar Macrophages Can Control Respiratory Syncytial Virus Infection in the Absence of Type I Interferons.
J Innate Immun.
2016; 8(5): 452–63. PubMed Abstract
| Publisher Full Text
- 53.
Sun Y, Jain D, Koziol-White CJ, et al.:
Immunostimulatory Defective Viral Genomes from Respiratory Syncytial Virus Promote a Strong Innate Antiviral Response during Infection in Mice and Humans.
PLoS Pathog.
2015; 11(9): e1005122. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 54.
Marr N, Turvey SE, Grandvaux N:
Pathogen recognition receptor crosstalk in respiratory syncytial virus sensing: a host and cell type perspective.
Trends Microbiol.
2013; 21(11): 568–74. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 55.
Kim TH, Lee HK:
Innate immune recognition of respiratory syncytial virus infection.
BMB Rep.
2014; 47(4): 184–91. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 56.
Kurt-Jones EA, Popova L, Kwinn L, et al.:
Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus.
Nat Immunol.
2000; 1(5): 398–401. PubMed Abstract
| Publisher Full Text
- 57.
Tal G, Mandelberg A, Dalal I, et al.:
Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease.
J Infect Dis.
2004; 189(11): 2057–63. PubMed Abstract
| Publisher Full Text
- 58.
Awomoyi AA, Rallabhandi P, Pollin TI, et al.:
Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children.
J Immunol.
2007; 179(5): 3171–7. PubMed Abstract
| Publisher Full Text
- 59.
Tulic MK, Hurrelbrink RJ, Prele CM, et al.:
TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide.
J Immunol.
2007; 179(1): 132–40. PubMed Abstract
| Publisher Full Text
- 60.
Haynes LM, Moore DD, Kurt-Jones EA, et al.:
Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus.
J Virol.
2001; 75(22): 10730–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 61.
Ehl S, Bischoff R, Ostler T, et al.:
The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus.
Eur J Immunol.
2004; 34(4): 1146–53. PubMed Abstract
| Publisher Full Text
- 62.
Caballero MT, Serra ME, Acosta PL, et al.:
TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization.
J Clin Invest.
2015; 125(2): 571–82. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 63.
Goritzka M, Pereira C, Makris S, et al.:
T cell responses are elicited against Respiratory Syncytial Virus in the absence of signalling through TLRs, RLRs and IL-1R/IL-18R.
Sci Rep.
2015; 5: 18533. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 64.
Zelenay S, Reis e Sousa C:
Adaptive immunity after cell death.
Trends Immunol.
2013; 34(7): 329–35. PubMed Abstract
| Publisher Full Text
- 65.
Hosakote YM, Brasier AR, Casola A, et al.:
Respiratory Syncytial Virus Infection Triggers Epithelial HMGB1 Release as a Damage-Associated Molecular Pattern Promoting a Monocytic Inflammatory Response.
J Virol.
2016; 90(21): 9618–31. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 66.
Foronjy RF, Ochieng PO, Salathe MA, et al.:
Protein tyrosine phosphatase 1B negatively regulates S100A9-mediated lung damage during respiratory syncytial virus exacerbations.
Mucosal Immunol.
2016; 9(5): 1317–29. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 67.
Lay MK, Bueno SM, Gálvez N, et al.:
New insights on the viral and host factors contributing to the airway pathogenesis caused by the respiratory syncytial virus.
Crit Rev Microbiol.
2016; 42(5): 800–12. PubMed Abstract
| Publisher Full Text
- 68.
Saravia J, You D, Shrestha B, et al.:
Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33.
PLoS Pathog.
2015; 11(10): e1005217. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 69.
McNamara PS, Ritson P, Selby A, et al.:
Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis.
Arch Dis Child.
2003; 88(10): 922–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 70.
Kim CK, Chung CY, Choi SJ, et al.:
Bronchoalveolar lavage cellular composition in acute asthma and acute bronchiolitis.
J Pediatr.
2000; 137(4): 517–22. PubMed Abstract
| Publisher Full Text
- 71.
Everard ML, Swarbrick A, Wrightham M, et al.:
Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection.
Arch Dis Child.
1994; 71(5): 428–32. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 72.
Geerdink RJ, Pillay J, Meyaard L, et al.:
Neutrophils in respiratory syncytial virus infection: A target for asthma prevention.
J Allergy Clin Immunol.
2015; 136(4): 838–47. PubMed Abstract
| Publisher Full Text
- 73.
Funchal GA, Jaeger N, Czepielewski RS, et al.:
Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils.
PLoS One.
2015; 10(4): e0124082. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 74.
Cortjens B, de Boer OJ, de Jong R, et al.:
Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease.
J Pathol.
2016; 238(3): 401–11. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 75.
McNamara PS, Flanagan BF, Hart CA, et al.:
Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis.
J Infect Dis.
2005; 191(8): 1225–32. PubMed Abstract
| Publisher Full Text
- 76.
Bertrand P, Lay MK, Piedimonte G, et al.:
Elevated IL-3 and IL-12p40 levels in the lower airway of infants with RSV-induced bronchiolitis correlate with recurrent wheezing.
Cytokine.
2015; 76(2): 417–23. PubMed Abstract
| Publisher Full Text
- 77.
Davidson S, Crotta S, McCabe TM, et al.:
Pathogenic potential of interferon αβ in acute influenza infection.
Nat Commun.
2014; 5: 3864. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 78.
Herold S, Steinmueller M, von Wulffen W, et al.:
Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand.
J Exp Med.
2008; 205(13): 3065–77. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 79.
Hussell T, Openshaw PJ:
Intracellular IFN-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection.
J Gen Virol.
1998; 79(Pt 11): 2593–601. PubMed Abstract
| Publisher Full Text
- 80.
Harker JA, Godlee A, Wahlsten JL, et al.:
Interleukin 18 coexpression during respiratory syncytial virus infection results in enhanced disease mediated by natural killer cells.
J Virol.
2010; 84(8): 4073–82. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 81.
Meng J, Stobart CC, Hotard AL, et al.:
An overview of respiratory syncytial virus.
PLoS Pathog.
2014; 10(4): e1004016. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 82.
Neyt K, Lambrecht BN:
The role of lung dendritic cell subsets in immunity to respiratory viruses.
Immunol Rev.
2013; 255(1): 57–67. PubMed Abstract
| Publisher Full Text
- 83.
Iwasaki A, Medzhitov R:
Control of adaptive immunity by the innate immune system.
Nat Immunol.
2015; 16(4): 343–53. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 84.
Roux X, Remot A, Petit-Camurdan A, et al.:
Neonatal lung immune responses show a shift of cytokines and transcription factors toward Th2 and a deficit in conventional and plasmacytoid dendritic cells.
Eur J Immunol.
2011; 41(10): 2852–61. PubMed Abstract
| Publisher Full Text
- 85.
Ruckwardt TJ, Malloy AM, Morabito KM, et al.:
Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response.
PLoS Pathog.
2014; 10(2): e1003934. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 86.
Kerrin A, Fitch P, Errington C, et al.:
Differential lower airway dendritic cell patterns may reveal distinct endotypes of RSV bronchiolitis.
Thorax.
2016; pii: thoraxjnl-2015-207358. PubMed Abstract
| Publisher Full Text
- 87.
Falsey AR, Singh HK, Walsh EE:
Serum antibody decay in adults following natural respiratory syncytial virus infection.
J Med Virol.
2006; 78(11): 1493–7. PubMed Abstract
| Publisher Full Text
- 88.
Chiu C, Openshaw PJ:
Antiviral B cell and T cell immunity in the lungs.
Nat Immunol.
2015; 16(1): 18–26. PubMed Abstract
| Publisher Full Text
- 89.
Habibi MS, Jozwik A, Makris S, et al.:
Impaired Antibody-mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus.
Am J Respir Crit Care Med.
2015; 191(9): 1040–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 90.
Vissers M, Ahout IM, de Jonge MI, et al.:
Mucosal IgG Levels Correlate Better with Respiratory Syncytial Virus Load and Inflammation than Plasma IgG Levels.
Clin Vaccine Immunol.
2015; 23(3): 243–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 91.
Jacobino SR, Nederend M, Hennus M, et al.:
Human amniotic fluid antibodies protect the neonate against respiratory syncytial virus infection.
J Allergy Clin Immunol.
2016; 138(5): 1477–1480.e5. PubMed Abstract
| Publisher Full Text
- 92.
Graham BS, Bunton LA, Wright PF, et al.:
Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice.
J Clin Invest.
1991; 88(3): 1026–33. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 93.
Openshaw PJ, Chiu C:
Protective and dysregulated T cell immunity in RSV infection.
Curr Opin Virol.
2013; 3(4): 468–74. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 94.
Legg JP, Hussain IR, Warner JA, et al.:
Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis.
Am J Respir Crit Care Med.
2003; 168(6): 633–9. PubMed Abstract
| Publisher Full Text
- 95.
Christiaansen AF, Knudson CJ, Weiss KA, et al.:
The CD4 T cell response to respiratory syncytial virus infection.
Immunol Res.
2014; 59(1–3): 109–17. PubMed Abstract
| Publisher Full Text
- 96.
Mangodt TC, van Herck MA, Nullens S, et al.:
The role of Th17 and Treg responses in the pathogenesis of RSV infection.
Pediatr Res.
2015; 78(5): 483–91. PubMed Abstract
| Publisher Full Text
- 97.
Park CO, Kupper TS:
The emerging role of resident memory T cells in protective immunity and inflammatory disease.
Nat Med.
2015; 21(7): 688–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 98.
Schenkel JM, Masopust D:
Tissue-resident memory T cells.
Immunity.
2014; 41(6): 886–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 99.
Knudson CJ, Weiss KA, Hartwig SM, et al.:
The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection.
J Virol.
2014; 88(16): 9010–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 100.
Jozwik A, Habibi MS, Paras A, et al.:
RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection.
Nat Commun.
2015; 6: 10224. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 101.
Sun L, Cornell TT, Levine A, et al.:
Dual role of interleukin-10 in the regulation of respiratory syncitial virus (RSV)-induced lung inflammation.
Clin Exp Immunol.
2013; 172(2): 263–79. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 102.
Weiss KA, Christiaansen AF, Fulton RB, et al.:
Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection.
J Immunol.
2011; 187(6): 3145–54. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 103.
Sun J, Cardani A, Sharma AK, et al.:
Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus.
PLoS Pathog.
2011; 7(8): e1002173. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 104.
Loebbermann J, Schnoeller C, Thornton H, et al.:
IL-10 regulates viral lung immunopathology during acute respiratory syncytial virus infection in mice.
PLoS One.
2012; 7(2): e32371. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 105.
Ouyang W, Rutz S, Crellin NK, et al.:
Regulation and functions of the IL-10 family of cytokines in inflammation and disease.
Annu Rev Immunol.
2011; 29: 71–109. PubMed Abstract
| Publisher Full Text
- 106.
Loebbermann J, Durant L, Thornton H, et al.:
Defective immunoregulation in RSV vaccine-augmented viral lung disease restored by selective chemoattraction of regulatory T cells.
Proc Natl Acad Sci U S A.
2013; 110(8): 2987–92. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 107.
Loebbermann J, Thornton H, Durant L, et al.:
Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection.
Mucosal Immunol.
2012; 5(2): 161–72. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 108.
Nagata DE, Ting H, Cavassani KA, et al.:
Epigenetic control of Foxp3 by SMYD3 H3K4 histone methyltransferase controls iTreg development and regulates pathogenic T-cell responses during pulmonary viral infection.
Mucosal Immunol.
2015; 8(5): 1131–43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 109.
Lee DC, Harker JA, Tregoning JS, et al.:
CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection.
J Virol.
2010; 84(17): 8790–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 110.
Fulton RB, Meyerholz DK, Varga SM:
Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection.
J Immunol.
2010; 185(4): 2382–92. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 111.
Ruckwardt TJ, Bonaparte KL, Nason MC, et al.:
Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities.
J Virol.
2009; 83(7): 3019–28. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 112.
Durant LR, Makris S, Voorburg CM, et al.:
Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice.
J Virol.
2013; 87(20): 10946–54. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 113.
Mazur NI, Martinón-Torres F, Baraldi E, et al.:
Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics.
Lancet Respir Med.
2015; 3(11): 888–900. PubMed Abstract
| Publisher Full Text
- 114.
Kim HW, Canchola JG, Brandt CD, et al.:
Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine.
Am J Epidemiol.
1969; 89(4): 422–34. PubMed Abstract
- 115.
Karron RA, Luongo C, Thumar B, et al.:
A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children.
Sci Transl Med.
2015; 7(312): 312ra175. PubMed Abstract
| Publisher Full Text
- 116.
Graham BS, Modjarrad K, McLellan JS:
Novel antigens for RSV vaccines.
Curr Opin Immunol.
2015; 35: 30–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 117.
The IMpact-RSV Study Group: Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants.
Pediatrics.
1998; 102(3): 531–7. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 118.
Boyoglu-Barnum S, Todd SO, Chirkova T, et al.:
An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice.
Virology.
2015; 483: 117–25. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 119.
Saso A, Kampmann B:
Vaccination against respiratory syncytial virus in pregnancy: a suitable tool to combat global infant morbidity and mortality?
Lancet Infect Dis.
2016; 16(8): e153–63. PubMed Abstract
| Publisher Full Text

Comments on this article Comments (0)