Keywords
Actin isoforms, monoclonal antibodies, epitope, muscle repair
This article is included in the Antibody Validations gateway.
Actin isoforms, monoclonal antibodies, epitope, muscle repair
Expression of actin isoforms in skeletal muscle at the mRNA and protein levels have been described since the 1980s using tissue extracts (Gunning et al., 1983; Hayward & Schwartz, 1986; Hayward et al., 1988; Minty et al., 1982; Ordahl, 1986; Paterson & Eldridge, 1984; Sassoon et al., 1988; Vandekerckhove et al., 1986). These studies have indicated that sarcomeric actins (α-CAA and α-SKA) are expressed in fetal and regenerating skeletal muscle, whereas α-SKA becomes the predominant actin isoform in mature skeletal muscle. Noteworthy, during fetal life, another actin isoform, α-SMA, is highly expressed and precedes the sarcomeric isoforms (Bochaton-Piallat et al., 1992; Hayward & Schwartz, 1986; McHugh et al., 1991; Woodcock-Mitchell et al., 1988).
Nevertheless, very little is known concerning the precise localization of these isoforms due to the lack of specific α-SKA and α-CAA antibodies. The first mAb against α-CAA has allowed the identification, by immunohistochemistry, of a α-CAA transient expression in human skeletal muscle satellite cells during skeletal regeneration induced by muscle injury, while normal skeletal muscle was negative (Franke et al., 1996). Ten years later, the same group, in a more extensive study, has further analyzed the expression and localization of α-CAA in normal, regenerating, diseased and neoplastic human muscle tissues (Moll et al., 2006). In human fetal skeletal muscle, a uniform strong α-CAA staining was observed while in normal adult skeletal muscle, α-CAA was identified only in few thin fibers. α-CAA staining of thin fibers became stronger in human regenerating skeletal muscle after traumatic injury, as well as in Duchenne muscular dystrophy (DMD). Whether the persistence of α-CAA in DMD myofibers is due to a lack of differentiation or to a regenerative process deserves further examination. Interestingly, resting satellite cells in healthy adult muscle lack this isoform, whereas, when satellite cells are activated during the regeneration process, α-CAA is up-regulated. Unfortunately, in these two studies, a comparative α-SKA staining was not performed.
In a more recent study, the switch of α-CAA to α-SKA during the differentiation of skeletal muscle from mouse embryonic stem cells has been examined (Mizuno et al., 2009). In this in vitro system, α-CAA appeared first in myoblasts, with no staining for α-SKA. During cell fusion, α-SKA appeared. When myotubes began to form sarcomers, α-SKA expression increased while α-CAA began to decrease. Finally, mature skeletal muscle fibers were mainly composed of α-SKA. Although, this in vitro system seems to recapitulate the in vivo skeletal muscle differentiation at the level of sarcomeric actins switching, the in vivo origin of progenitor cells during in vivo muscle differentiation/repair remains elusive.
The sequential expression of the two striated actins during: i) heart development (high expression of α-SKA at birth, predominant expression of α-CAA in differentiated cardiac muscle at adult life) and ii) skeletal muscle development (high expression of α-CAA at birth, predominant of α-SKA in differentiated skeletal muscle at adult life) has been known from mRNA studies for decades, but little is known about the distribution and/or the localization of the two α-sarcomeric muscle actins, because of the lack of double immunostainings availability. Nevertheless, our laboratory, in collaboration with others, has studied α-SKA expression and distribution, in particular in developing and pathological hearts, using affinity polyclonal antibodies (Clément et al., 1999), again without double immunostaining.
As mentioned above, α-CAA distribution has been studied during muscle development and repair (Moll et al., 2006), but a comparative study with α-SKA was still missing. Our mAbs were raised using the acetylated N-terminus decapeptide of each isoform (see Table 1). The two different mAb subtypes (anti-α-SKA, IgG2b and anti-α-CAA, IgG1), allowed a clear analysis of the expression and distribution of the two actin isoforms in mature skeletal muscle and during regeneration after micro-lesions by means of highly specific anti- mouse subtype secondary antibodies.
The epitope recognized by each antibody is enlightened in bold.
| Actin isoform | Immunogen | MAb name | Clone | Subtype | Original Reference | Distributors | Catalog Number |
|---|---|---|---|---|---|---|---|
| α-SMA | AcEEEDSTALVC | Anti-α-SM1 | 1A4 | IgG2a | Skalli et al., 1986 | Abcam AbD Serotec Cell Marque Corporation Dako eBioscience EMD Millipore Genemed Biotechnologies Nordic-MUbio R§D Systems Santa Cruz Biotechnology Sigma-Aldrich Spring Bioscience Zeta Corporation | ab-7817 MCA5781GA 202M M0851 14–9760 113200 61–0001 MUB0107S MAB1420 sc-32251 A5228 E14344 Z2066 |
| γ-SMA | AcEEETTALVC | Anti-γ-SMA | 20D2 | IgG1 | Arnoldi et al., 2013 | NA | NA |
| β-CYA | AcDDDIAALVC | Anti-β-CYA | 4C2 | IgG1 | Dugina et al., 2009 | AbD Serotec EMD Millipore Nordic-MUbio | MCA5775GA MABT825 MUB0110S |
| γ-CYA | AcEEEIAALVC | Anti-γ-CYA | 2A3 | IgG2b | Dugina et al., 2009 | AbD Serotec EMD Millipore Nordic-MUbio | MCA5776GA MABT824 MUB0111S |
| α-SKA and α-CAA Sarcomeric actins | Rabbit skeletal actin | Anti-α-SR1 | 5C5 | IgM | Skalli et al., 1988 | Dako Santa Cruz Biotechnology Sigma-Aldrich Spring Bioscience | M0874 sc-58670 A 2172 E16654 |
| α-SKA | AcDEDETTALVC | Anti-α-SKA | 3B3 | IgG1 | Driesen et al., 2009 | Nordic-MUbio | MUB0108S |
| α-SKA | AcDEDETTALVC | Anti-α-SKA | 10D2 | IgG2a | Driesen et al., 2009 | NA | NA |
| α-CAA | AcDDEETTALVC | Anti-α-CAA | 22D3 | IgG1 | Driesen et al., 2009 | EMD Millipore Nordic-MUbio | MABT823 MUB0109S |
Details of all reagents with reference to the immunoblot and immunostaining procedure can be found in Table 2 and Table 3. Crucial are the conditions of fixation and permeabilization for relevant immunostaining. With cells in culture as well as with tissues, we have tested a large number of conditions such as MeOH, EtOH, PFA-TX100. By far, the use of PFA, followed by MeOH, as described in Table 3 and previously defined (Dugina et al., 2009), allowed the best detection of every actin isoform, likely because of availability of the actin molecule N-terminus.
| Protocol steps | Reagent | Concentration/ dilution | Time |
|---|---|---|---|
| Migration of Purified α-SKA and α-CAA on SDS-PAGE | 1μg/lane | overnight | |
| • Transfer on Nitrocellulose membranes. • Membranes cut in strips for Ab-peptides incubation. | 2 h | ||
| Preincubation of antibodies with peptides | MAb: • anti-α-SKA (10D2) • anti-α-CAA (22D3) For details, see Table 1 Blocking Peptides (see Figure 1) | 1:5000 1:5000 100 μg/ml | 1 h |
| Membranes pretreatment | 5% dried skimmed milk in TBS | 5% | 1 h |
| Membranes Incubation with antibody-peptides | In 0.1% TX-100/TBS | 2 h | |
| Membranes Incubation with 2nd antibody | Goat Anti-Mouse IgG (H+L) HRP Conjugate (BioRad) #1706516 In 0.1% TX-100/TBS | 1:10’000 | 1 h |
| HRP activity development | ECL protocol according to (RPN 2109; GE Healthcare), | 5–60 sec |
| Protocol steps | Reagent | Concentration /dilution | Time | Catalogue Number |
|---|---|---|---|---|
| • Cryopreserved Rat muscles • Cryostat sections (3μm) | ||||
| Fixation | • Paraformaldehyde • Methanol | 1% 100% | 30 min at RT 5 min at –20°C | |
| Staining | Primary Abs: MAbs: • anti-α-SKA (10D2) • anti-α-CAA (22D3) • anti-α-SMA (1A4) For details, see Table 1 • anti-vimentin (clone V9) Immunogen: Purified vimentin from porcine eye lens Specificity: the antibody cross-reacts with vimentin in man, cow, dog, hamster, horse, rhesus and African green monkey, rabbit, rat and rat kangaroo. No cross-reaction with mouse vimentin could be demonstrated, and results on chicken specimens are contradictory. Rabbit polyclonal Ab: • anti-α-CAA (Clément et al., 2003) | 1:50 1:50 1:50 1:300 1:100 | 2 h | Home made Home made Home made Dako #M 0725 Home made |
| Secondary Abs (Jackson Immmunoresearch): • AffiniPure Goat Anti-Mouse IgG1, Alexa 488 • AffiniPure Goat Anti-Mouse IgG1, Alexa 594 • AffiniPure Goat Anti-Mouse IgG2a, Alexa 488 • AffiniPure Goat Anti-Mouse IgG2a, Alexa 594 • AffiniPure Goat Anti-Rabbit-Alexa 594 | 1:200 1:200 1:200 1:200 1:100 | 1 h | 115-545-205 115-585-205 115-545-206 115-585-206 111-585-144 |
All animal experiments (production of antibody in mice, rat wounds, tissue samplings) were approved by and performed in accordance with the cantonal and federal veterinary authorities.
Tibialis muscle specimens from female Wistar rats older than 10 weeks were rapidly embedded in OCT compound (Tissue-Tek), snap-frozen in a beaker containing precooled liquid isopentane, immerged in liquid nitrogen, and stored at -80°C. For muscle repair studies, rat tibialis muscles were injured with a liquid nitrogen cooled needle, using a well-set simple rat model (Rocheteau et al., 2012). Muscles were taken, OCT embedded and frozen at different days after injury.
MAbs against α-SKA and α-CAA were prepared following the Repetitive Immunizations Multiple Sites (RIMMS) strategy (Kilpatrick et al., 1997). This strategy uses lymphocytes from regional draining lymph nodes and an immunization schedule significantly shorter than conventional techniques: two weeks instead of several months. Along with the use of less antigen, this approach allows to obtain hybridomas in a month.
Mice were immunized with the acetylated N-terminal decapeptide of α-SKA (Ac-DEDETTALVC-COOH) or α-CAA (Ac-DDEETTALVC-COOH) conjugated with keyhole limpet haemocyanin through the cysteine peptide C-terminus (KLH, Imject Maleimide Activated carrier proteins, Pierce) according to the instructions of the manufacturer. Briefly, over a period of 10 days, 5 injections of 5 μg of protein (α-SKA or α-CAA peptide x KLH) emulsified in complete Freund’s adjuvant (first injection) or with incomplete Freund’s adjuvant (for the remaining injections) and RIBI adjuvants (Sigma-Aldrich) were given at six subcutaneous sites proximal to draining peripheral lymph nodes (PLNs) in three anesthetized six-week-old female BALB/c mice (200μl/mice). Two days after the final boosts, animals were sacrificed. PLNs were harvested from popliteal, superficial inguinal, axillary, and brachial lymph nodes and dissociated. Up to 1 × 108 lymphocytes per 3 mice, were used for fusions with 2.5 × 107 with NSO myeloma cells using 50% polyethylene glycol (PEG 1500, Sigma-Aldrich). Fused cells were plated in 24-well tissue culture plates (4000 cells/well). Hybridomas were grown in DMEM+ pyruvate, 5% FCS, 10% NCTC 109 (Sigma-Aldrich), 1× MEM Non-Essential Amino Acids (Gibco), 1% Hybridoma Fusion and Cloning Supplement (HFCS, Roche), penicillin/streptomycin, and 1× selective media hypoxanthine/aminopterin/thymidine (HAT). Supernatants were harvested after 2 weeks and screened for antibody specificity by ELISA and immunofluorescence staining (see below). Hybridomas from wells of interest were distributed into 96-well plates (1-2-5-10 cells/well) for the first limited dilution using 1× hypoxanthine and thymidine (HT) in place of HAT. After about 10 days of culture, wells with single clones were identified by microscope and their supernatants were harvested for screening. Positive clones were re-plated using another limited dilution for a further 10 days in DMEM containing 10% FCS. Clones secreting the specific mAb were expanded and frozen.
Supernatants of hybridoma cells secreting anti-α-SKA or anti-α-CAA were screened by: i) triple ELISA, using 96 well plates coated with α-SMA, α-SKA and α-CAA BSA-conjugated peptides, using Maleimide Activated BSA (Pierce) according to the instructions of the manufacturer; ii) Western blotting using platelets, heart, aorta, skeletal muscle, gizzard extracts (Driesen et al., 2009); iii) immunofluorescence using rat lip, skeletal and heart sections. Selected hybridomas were cloned twice by limited dilution, as described above, and the final mAB characterization was performed by immunoblotting after transfer of one-dimensional gels containing tissue and cell extracts and finally by blocking assays (Chaponnier et al., 1995) using the N-terminal peptides of α-SKA and α-CAA.
Purified α-SKA (Spudich & Watt, 1971) and α-CAA (Zot & Potter, 1981) were run on 10% SDS-PAGE (Laemmli, 1970) and electroblotted to nitrocellulose according to Towbin et al. (Towbin et al., 1979). After preincubation with the respective different length peptides (listed in Figure 1), the antibodies, diluted in Tris-buffered saline (TBS) solution containing 3% BSA and 0.1% Triton X-100, were incubated on membranes strips for two hours at room temperature. After 3 washes with TBS, a second incubation was performed with peroxidase-conjugated affinity purified goat anti-rabbit IgG (Biorad) at a dilution of 1:10,000 in TBS containing 0.1% BSA and 0.1% Triton X-100. Peroxidase activity was developed using the ECL Western blotting system (GE Healthcare), exposed to AX Konica Minolta films for 5–60 sec, and processed with Curix-60 developing machine (Agfa). Blots were scanned and quantified using densitometric analysis ImageJ v1.49 software (NIH, http://rsb.info.nih.gov/ij/).
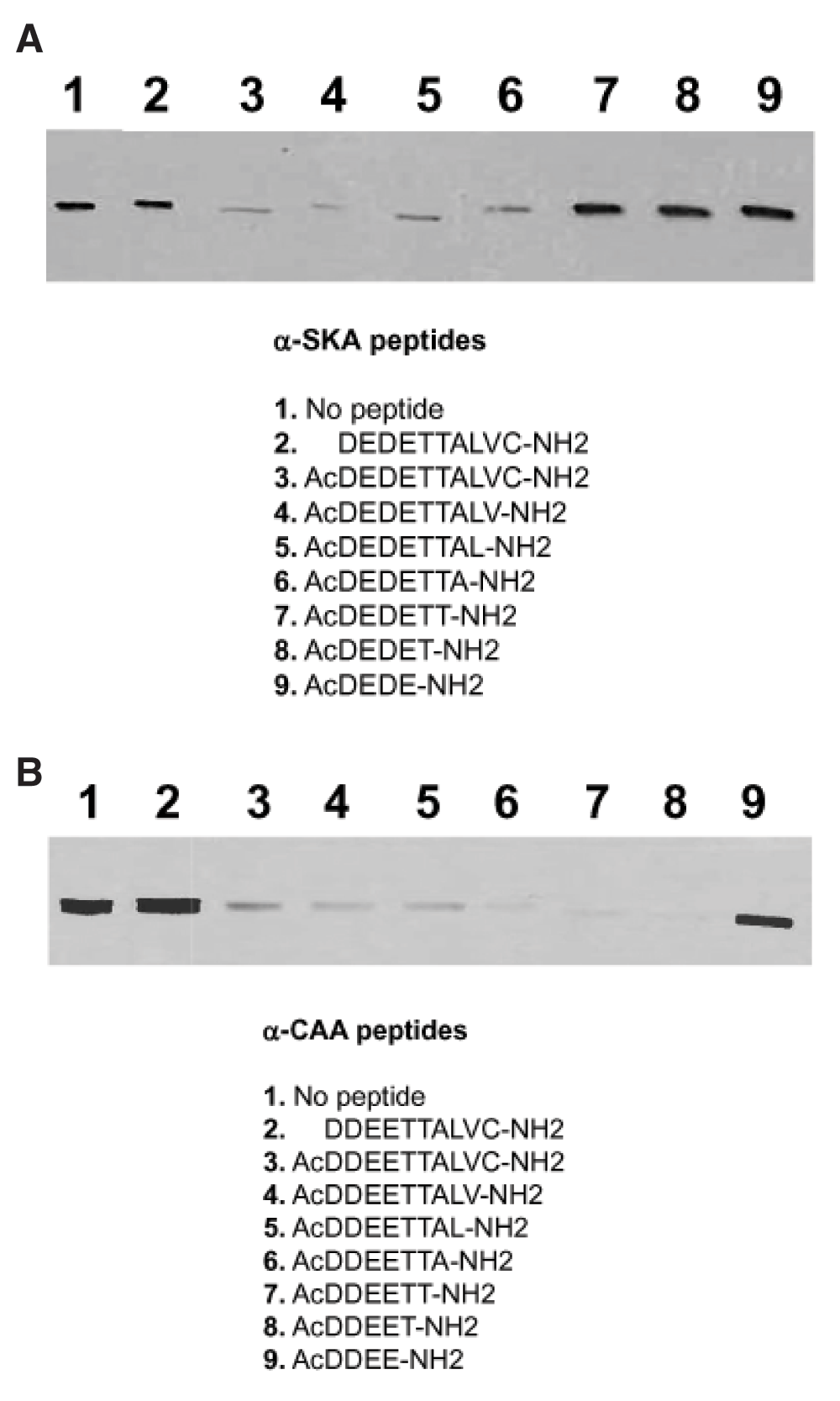
Purified α-SKA (A) or α-CAA (B) was run on 10% SDS-PAGE and transferred on nitrocellulose membrane. Membrane strips were incubated with the mAb anti-α-SKA (A) or anti-α-CAA (B) alone (control, lane 1) or mixed with the listed peptides (2–9). The epitope recognized by anti-α-SKA (A) includes the acetyl group and the first 7 amino acids of the α-SKA sequence. The epitope recognized by anti-α-CAA (B) includes the acetyl group and the first 5 amino acids of the α-CAA sequence.
Cryopreserved tissues were sliced (3 μm) with a cryostat microtome (Microm). Sections were positionned on glass slides, fixed with 1% PFA for 30 min at room temperature, followed by three washes with PBS and a 3 min treatment with methanol at -20°C. After three 5 min washes with PBS at RT, tissue sections on glass slides were incubated with primary (1h) and secondary antibodies (30 min) at appropriate dilutions (see Table 3). Normal rat serum (1:50) was used to block non-specific sites, and DAPI for nuclear staining. After washing in PBS, sections on slides were mounted in polyvinyl alcohol (PVA: 50 mM Tris-phosphate pH 9.0, 0.1% chlorobutanol, 20% polyvinyl alcohol, 0.5‰ phenol red, 20% glycerol) (Lennette, 1978). Images were acquired using a Zeiss Axiophot microscope (Carl Zeiss), equipped with plan apochromatic 10x, 20x, or 40x objectives and a high sensibility color camera (Axiocam, Zeiss).
After a careful selection of highly specific secondary antibodies against mouse subtypes, testing a large panel of these antibodies, we selected those commercialized by Jackson Immunoresearch and Southern Biotechnology.
Acetylated N-terminal peptide of different length (4–10 amino acids) and non-acetylated N-terminal decapeptide of α-SKA and α-CAA were tested for their blocking ability of the respective mAb (Figure 1). We identified the epitope of α-SKA (AcDEDETTA) and of α-CAA (AcDDEET). The epitope of the other actin isoforms were previously identified and are listed in Table 1 (in bold letters). Noteworthy, the acetyl group is a critical element of the epitope of each isoform.
The normal myonuclear turnover in rodents is estimated at 1–2% per week (Schmalbruch & Lewis, 2000). As a first investigation, we have compared the distribution of α-CAA with α-SKA, α-SMA and vimentin on cryostat sections of adult rat tibialis muscle. Although a minority of myocytes expressed α-CAA, we have observed two types of α-CAA positive cells in skeletal fibers: one was mainly located in interstitial connective tissue (Figure 2A, a–c, d–f) and the other was localized in the muscle mass (Figure 2A, g–i, arrowhead). The first type corresponded most likely the “muscle spindles”, described by Moll et al. (Moll et al., 2006), as “modified muscle fibers of neuromuscular spindles, believed to act as sensors of muscle tension”. The “spindle” cells expressed exclusively α-CAA, whereas the “classical muscle fibers”, in addition to a high expression of α-CAA, displayed α-SKA at various levels (Figure 2A, g–i, j–l), probably according to the state of differentiation during self-renewal of myocytes.
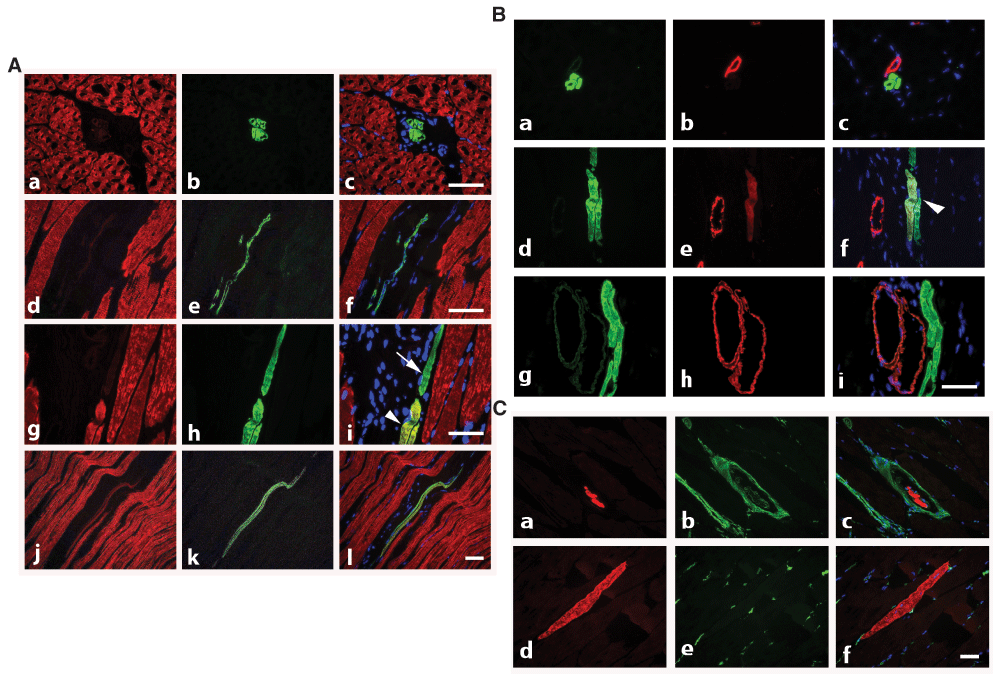
A) Co-staining with anti-α-SKA (red) and anti-α-CAA (green) allows the detection of isolated thin α-CAA positive fibers in interstitial connective tissue (transversal sections, a–c: longitudinal section, d–f) and of renewing muscle fibers expressing both isoforms (longitudinal section, g–l, arrowhead), or only α-CAA (arrow). Merged images are shown on right column. Bars = 50 μm. B) Co-staining with anti-α-CAA (green) and anti-α-SMA (red) shows that α-CAA positive spindle cells are in close connection with α-SMA positive vessel (a–c), that early muscle fiber self-renewal is characterized by co-expression of both isoform (d–f, arrowhead), although more advanced regenerated fibers are only α-CAA positive (g–i). Merged images are shown on right column. Bar = 50 μm. C) Co-staining with anti-α-CAA (red) and anti-vimentin (green) allows the detection of α-CAA positive muscle spindle cells surrounded by a capsule containing vimentin positive cells (transversal section, a–c) and of renewing α-CAA positive fibers in contact with vimentin positive cells (longitudinal section, d–f). Merged images are shown on right column. Bar = 50 μm.
It is well known that during skeletal muscle development, α-SMA is the first muscle actin to be expressed in myocytes during fetal life. Therefore, we also investigated the expression of α-SMA during muscle self-renewal. We observed that a few regenerating α-CAA positive fibers displayed α-SMA (Figure 2B, d–f).
As vimentin is expressed in the capsule of muscle spindle cells (Cizkova et al., 2009), we investigated further whether the α-CAA positive fibers located in interstitial spaces could be identified as component of muscle spindles. After co-staining of muscle sections for α-CAA and vimentin, we confirmed that a capsule containing vimentin-positive cells surrounded isolated α-CAA positive spindle fibers (Figure 2C, a–c). Noteworthy, renovating fibers displayed contacts with vimentin positive cells (Figure 2C, d–f).
The use of specific mAbs against actin isoforms allows the tracking and follow-up of myocytes renewal during the muscle repair process. In particular, complete and fast repair can be observed after muscle micro-lesions obtained after light injury induced by nitrogen-cooled needle application. At early stage (4 day post-injury), an important population of α-CAA positive myofibers was observed (Figure 3A) at injury sites. These cells, being α-SKA negative or marginally positive, are probably in an early stage of differentiation. Few of them co-expressed α-SMA (Figure 3C, a–c, arrowhead). Only rarely, α-SMA positive myofibroblasts, the hallmark of fibrotic process (Tomasek et al., 2002) were detected (Figure 3C, a–c, arrow). At 5d post-injury, fibers started to express more importantly α-SKA in addition to α-CAA in the regenerating location (Figure 3B, a–f). In our model, muscle regeneration was very rapid, as expression of α-SMA was not any longer detectable 5d after injury (Figure 3C, d–f). At 9d post-injury, co-expression of α-SKA and α-CAA became rare (Figure 3B, g–i). These results suggest that skeletal muscle has a high capacity of regeneration after micro-injury and that actin isoform specific mAbs represent an important tool for muscle regeneration tracking.
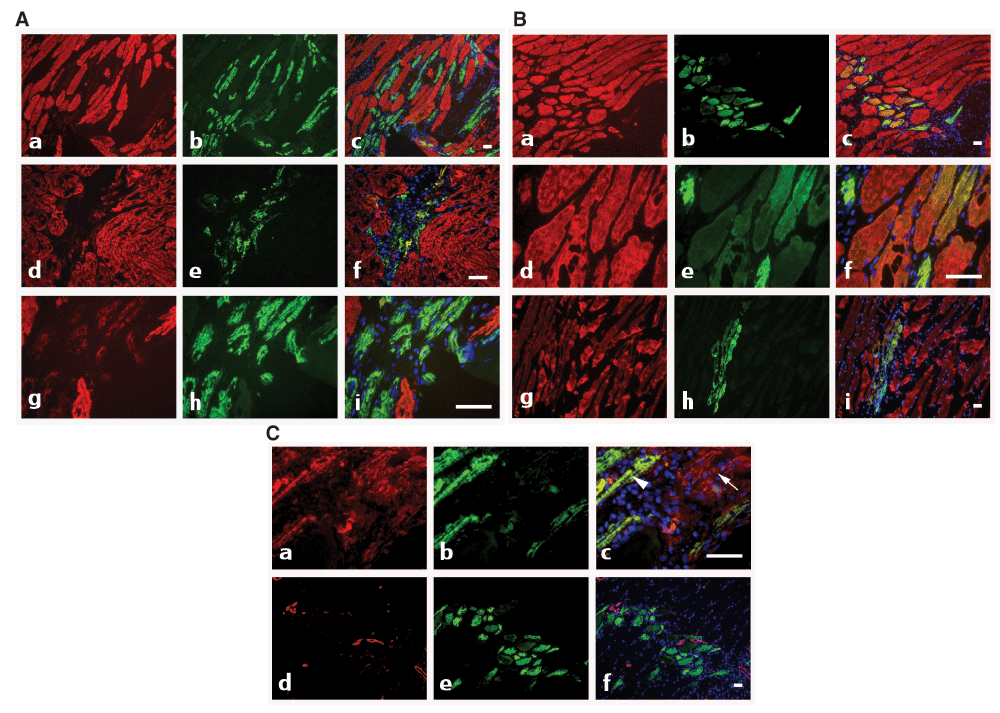
A) At 4d post-injury, co-staining with anti-α-SKA (red) and anti-α-CAA (green) shows the presence of α-CAA positive fibers in the injured muscle area (a–i). At this stage of regeneration, only a few fibers co-express both isoforms (d–i), with a low α-SKA level (g–i). Merged images are shown on right column. Bars = 50 μm. B) At 5d–9d post-injury, co-staining with anti-α-SKA (red) and anti-α-CAA (green) shows that after 5d, most fibers co-express both isoforms (a–f), whereas at 9d (g–i), α-SKA positive fibers become predominant. Merged images are shown on right column. Bars = 50 μm. C) At 4d–5d post-injury, co-staining with anti-α-SMA (red) and anti-α-CAA (green) shows that during the healing process, only a few fibers co-express both isoforms after 4d (a–c, arrowhead), whereas after 5d, only α-CAA positive fibers are detected (d–f). After 4d, myofibroblasts might participate to the repair process and are detected by using the anti-α- SMA mAb (a–c, arrow). Merged images are shown on right column. Bars = 50 μm.
In conclusion, α-CAA, in conjunction with the expression of α-SMA and α-SKA, appears to represent a valuable marker for the identification of myofibers renewal in skeletal muscle and for the analysis of the degree of fiber differentiation. For this purpose, it is important to use well-characterized and specific antibodies. Furthermore, high quality anti-mouse subtype secondary antibodies allow double immunostaining necessary for this type of investigation.
F1000Research: Dataset 1. Raw data for Figures 1a, b., 10.5256/f1000research.8154.d117164 (Chaponnier & Gabbiani, 2016).
The authors sell the anti-actin isoform antibodies to companies through Unitec, University of Geneva, unitec@unige.ch
This work was supported by the Swiss National Science Foundation (grant number 310030_125320) to C.C.
We thank Anita Hiltbrunner and Aman Ahmed Mohamed, for their high quality technical assistance, Dr. Marie-Luce Bochaton-Piallat for advice and help with Photoshop and Dr. Stéphane König, Department of Basic Neurosciences, for advice and discussion about the muscle micro-lesion model.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
References
1. Gunning PW, Ghoshdastider U, Whitaker S, Popp D, et al.: The evolution of compositionally and functionally distinct actin filaments.J Cell Sci. 2015; 128 (11): 2009-19 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 01 Jun 16 |
read | ||
|
Version 1 30 Mar 16 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Your table 1 is very useful in bringing together lots of information about antibodies that are being resold by many vendors. This is critical information that ... Continue reading Dear Drs. Chaponnier and Gabbiani,
Your table 1 is very useful in bringing together lots of information about antibodies that are being resold by many vendors. This is critical information that we will code into the antibodyregistry.org to help future authors avoid mistakes in cross-validation.
However, it seems that you have not registered your new antibodies with the antibodyregistry.org. This step is recommended by the instructions to authors at F1000 and also NIH grantees starting in May 2016, please see: http://f1000.com/resources/F1000Research-Antibody-Validation-Article-Template.docx
For the Dako antibody, the reference is most likely Dako Cat# M0725, RRID:AB_10013485, but please search the antibodyregistry.org to verify.
Your table 1 is very useful in bringing together lots of information about antibodies that are being resold by many vendors. This is critical information that we will code into the antibodyregistry.org to help future authors avoid mistakes in cross-validation.
However, it seems that you have not registered your new antibodies with the antibodyregistry.org. This step is recommended by the instructions to authors at F1000 and also NIH grantees starting in May 2016, please see: http://f1000.com/resources/F1000Research-Antibody-Validation-Article-Template.docx
For the Dako antibody, the reference is most likely Dako Cat# M0725, RRID:AB_10013485, but please search the antibodyregistry.org to verify.