Keywords
IBS, IBS-C, Lovastatin, homology modeling, multi-site docking
IBS, IBS-C, Lovastatin, homology modeling, multi-site docking
Irritable bowel syndrome (IBS) affects as many as 45 million people in the United States, and up to 23% of the worldwide population1. Depending on the region, as many as 43.3% of these patients will have irritable bowel syndrome with constipation (IBS-C)2. The illness affects both men and women; however, two-thirds of diagnosed sufferers are women. Studies have linked methane production to the pathogenesis of constipation and IBS, as well as obesity3. Methanogens – i.e. anaerobes that respire hydrogen to produce methane - are found in many habitats supporting anaerobic biodegradation of organic compounds, including human and animal intestinal tracts4,5. Archaea are the only confirmed, naturally occurring biological sources of methane. Methanobrevibacter smithii (M. smithii) is the predominant methanogen in the human intestine accounting for 94% of the methanogen population3.
The isoprenoid biosynthesis for the main cell membrane components in archaea (archaeol) relies on the same enzyme that catalyzes the biosynthesis of the isoprenoid cholesterol in humans - HMG-CoA reductase (mevalonate pathway)6. It has been previously suggested that statins, i.e. known HMG-CoA reductase inhibitors, can also interfere with the biosynthesis of the archaeal cell membrane and thus inhibit archaeal growth7. Statins, specifically lovastatin, have been shown to lower methanogenesis in human stool samples8 and can inhibit archaeal cell membrane biosynthesis without affecting bacterial numbers as demonstrated in livestock and humans. Lovastatin is a secondary metabolite produced during fungal growth and is found in oyster mushrooms9, red yeast rice10, and Pu-erh11.
Humans and archaea utilize the HMGR-I isoform for isoprenoid biosynthesis12. Mevastatin and lovastatin were both shown to inhibit growth of several rumen Methanobrevibacter isolates in the ~10 nmol/ml range3. While it is believed that statins inhibit methane production via their effect on cell membrane biosynthesis mediated by inhibition of HMG-CoA reductase, there is accumulating evidence for an alternative or additional mechanism of action where statins inhibit methanogenesis directly13. In one case, in silico molecular docking of the methanogenic enzyme F420-dependent NADP oxidoreductase (fno) showed that both lovastatin and mevastatin had higher affinities for the F420 binding site on fno than did F420 itself. It has been suggested that lovastatin may act as an inhibitor of fno14.
Several reviews have appeared describing the reduction of CO2 to CH4 in methanoarchaea15. Considering other mechanisms by which statins may inhibit methanogenesis directly, we have explored two important dehydrogenases in the main methanogenesis pathway, including F420-dependent methylenetetrahydromethanopterin dehydrogenase of M. smithii [A5UMI1- 275 amino acid residues], and evolutionarily related F420-dependent methylenetetrahydromethanopterin (methylene-H(4)MPT) dehydrogenase (mtd) of Methanopyrus kandleri [Q02394 – 358 amino acid residues]. Both only leverage F420 as a coenzyme, which assisted our computational analyses by avoiding issues associated with an NADP induced fit. The A5UMI1 sequence does not have crystallographic structural information in the Protein Data Bank (PDB)16, so we needed to identify acceptable templates to model this sequence. The Q02394 sequence, however matched the 3IQZ co-complex with methylenetetrahydromethanopterin (H4M) having 52% sequence homology. While both sequences required modeling, we needed to identify one or more acceptable templates for A5UMI1. After modeling and receptor site identification, we docked and rank-ordered multiple ligand variations across several modeled receptor sites to evaluate preferential binding characteristics for the ligands in question.
Protein sequences were extracted from UniProt17. Many protein structure modeling methods have been developed and are available with most performing well given crystallographic template(s) sharing sufficient sequence homology with target sequences of interest18. The Eidogen StructFast19,20 technology is well suited for this type of modeling. StructFast can operate in an automated mode where the best PDB template is automatically selected, or in a directed mode where modeling is guided based on a suggested PDB template21,22.
Once models for A5UMI1 and Q02394 were developed with StructFast, ligand binding sites were identified by inference from the respective PDB templates used in modeling and from the Eidogen SiteSeeker algorithm23. SiteSeeker looks for concave, surface features sufficiently exposed to enable ligand binding while also considering evolutionary conservation of sequence. In addition to sites identified by SiteSeeker, other sites were manually inferred within PyMOL v1.8 after aligning models and templates containing their respective co-complexed ligands. Residues on model structures with a 7Å cutoff of co-complexed ligands within the templates were exported and also processed as sites.
Ligands were carefully prepared considering different protonation states, isomers, and tautomers. We standardized charges, added missing hydrogens, enumerated ionization states, ionized functional groups, generated tautomers and isomers, and generated starting-point 3D coordinates for each ligand using BIOVIA’s (Accelrys’) Pipeline Pilot technology v8.524. Ligands were finally prepared into mol2 format25. Each representation was then docked into each identified site and scored using AutoDock Vina v1.1.226, an open docking technology that utilizes grid-based energy evaluation and efficient search of ligand torsional freedom.
The AutoDock Vina system requires that receptor site files be formatted in the PDBQT [Protein Data Bank, Partial Charge (Q), & Atom Type (T)] molecular structure file format. The MGLTools v1.5.427 were used for this file format conversion. Additionally, AutoDock Vina requires a defined grid box surrounding the receptor site residues. Here, we identified the center of mass of each receptor site using all atoms in the receptor site PDB file. We then calculated within Pipeline Pilot the maximum distance between any atom in the receptor site and the centroid in each x,y,z-direction. The lengths of each grid box were configured with these maximums. To insure reproducibility and comparability of docking simulations, we initiated each AutoDock Vina run with the same random seed value of 1162467901.
We identified three different PDB templates that had sufficient sequence homology to model the Q02394 sequence, by identifying other PDB co-complexes containing ligands with high 2D similarity to the H4M ligand. The top three PDBs showing significant sequence homology to Q02394 included: 3F47 (57%), 3H65 (57%), and 4JJF (52%). Each template was used to model Q02394. In each case, ligand-binding sites were readily inferred from the ligand binding sites found in the respective template structures.
The modeling of sequence A5UMI1 was straightforward given its high 52% sequence homology to 3IQZ. Each 3IQZ chain (A-F) was considered, given the possibility that one template-chain might offer additional or different insight into possible ligand binding locations. The Eidogen SiteSeeker algorithm identified only one site when template chains A, C, D were used, while two sites were identified in models leveraging template chains B, E, F. Unfortunately, the H4M site from the 3IQZ template was not easily inferred into any of the A5UMI1/3IQZ-based models, because 3IQZ has multiple chains involved in H4M binding.
Modeling sequences from PDB templates is done with individual chains. Quaternary modeling using models of individual chains can be very challenging. We manually modeled the H4M site as described in Figure 1. Since our aim was to dock all ligands across all possible ligand binding sites, we included the sites identified by inference (i.e. where ligands were present in templates), by the SiteSeeker algorithm run across single chain models, and by manually modeled sites as described by Figure 1. A total of 10 ligand-binding sites (Table 1) were identified across all the Q02394 and A5UMI1 models.
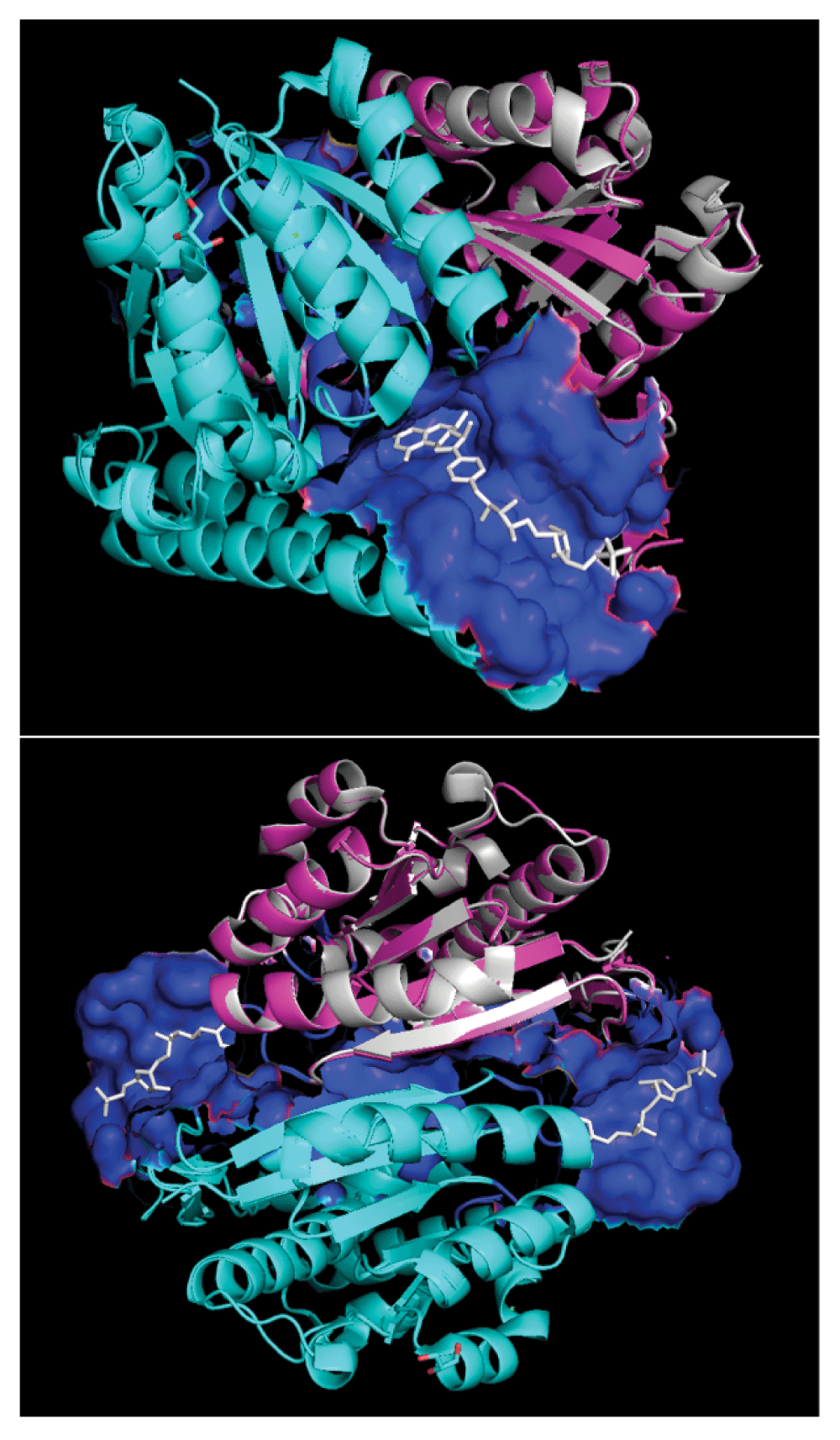
Modeled quaternary structure of A5UMI1/3IQZB (cyan) and A5UMI1/3IQZF (pink) after respective alignments onto chain-B and chain-F of 3IQZ within PyMOL28. 3IQZ’s chain-F is highlighted in silver. Dual chain model site residues (blue surface) were inferred from residues in chain-B and chain-F models that are within 7 Å of the 3IQZ ligand (H4M - white). 3IQZ’s chain-B and chain-F form a quaternary structure with two different H4M binding sites (bottom).
Four sites from the A5UMI1 modeling and six sites from Q02394 modeling were used in the docking simulations.
| A5UMI1 | Q02394 |
|---|---|
| 3IQZB (H4M 7Å), 1 chain | 3F47 (I2C) |
| 3IQZB (SiteSeeker1) | 3F47 (SiteSeeker) |
| 3IQZB (SiteSeeker2) | 3H65 (H4M) |
| 3IQZB (H4M)_3IQZF (7Å) | 3H65 (I2C) |
| 4JJF (FE9) | |
| 4JJF (SiteSeeker) |
The key ligands for this effort included lovastatin (lactone and hydroxyacid forms), F420, and simvastatin (lactone and hydroxyacid forms) and processed ligands that were found in the PDB templates used to model each sequence: 803, F42, H4M, I2C, FE9, SIM, 116, HMG, and 882. The latter four (SIM, 116, HMG, 882) were ligands found in the positive control templates for completeness.
The PDB often contains problematic ligand structures, so we processed both PDB ligands and ligands extracted from PubChem29 for lovastatin (lactone and hydroxyacid forms), F420, and simvastatin (lactone and hydroxyacid forms). It should be noted, the PDB considers 803 as lovastatin (lactone form), F42 as coenzyme-F420, and SIM as simvastatin (hydroxyacid form), though their actual structural forms may vary depending on the PDB entry. This is why we also use PubChem structural representations for lovastatin, F420, and simvastatin.
It is well established that the β-hydroxyacid form and not the closed-ring lactone form of lovastatin is the active HMGR-binding form of the molecule30. Simvastatin and lovastatin are commercially available in the lactone form; they behave as prodrugs which inhibit HMGR only after the opening of the lactone ring into the hydroxyacid form31,32. The degree of hydrophobicity of imidazole derivatives correlates with improved activity against human methanogenic archaea33.
Each ligand was computationally processed in the same way prior to docking. BIOVIA’s (Accelrys’) Pipeline Pilot was used for this ligand preparation. First, stereochemistry and charges were standardized, then ionized at pH 7.4, then tautomers (if present) were enumerated, and finally initial 3D models were determined. AutoDock Vina explores ligand 3D conformation, so the initial 3D models were simple starting points. Additionally, ligands were processed without the above standardization, ionization, and tautomer exploration. Each ligand representation was considered in the docking runs. Ligands processed with the standardization sequenced contained the prefix “STD_,” and ligands without standardization contained the prefix “RAW_.” Together, these expanded ligand representations can help gauge the docking algorithm’s sensitivity to the ligand’s structural representation.
A total of 88 ligand variations were systematically docked into the 10 identified binding sites across all the A5UMI1 and Q02394 models for a total of 880 docking simulations. Even though AutoDock Vina achieves two orders of magnitude speed-up and significantly improves the accuracy of the binding mode predictions compared to AutoDock 4, 880 docking simulations could have taken several weeks to complete. To accelerate the effort, we requisitioned a compute cluster in the Amazon EC234 cloud environment for approximately three days at a cost under $60.
The docking process scores ligand conformations based on ligand conformation and ligand-to-receptor interactions within a grid box. After the 880 docking simulations were complete, we rescored all docked ligand variations against their respective full model structures. This enabled a more realistic rank ordering given possible overlap with a docked ligand and other portions of a model not represented in the rectangular box. This also served as an internal control, since rescoring was completed independently of the docking simulations.
Since it is unknown which (if any site) might actually engage the ligands of interest, we calculated the average, minimum, and maximum affinity of each ligand/variation for each of the 10 sites. The top-two sites (highlighted in bold in Table 2) were used to then rank order each ligand. Table 3 shows the rank ordered ligands using the AutoDock Vina overall score, which considers steric interactions (Gauss 1, Gauss 2, and steric), dispersion/repulsion, hydrophobic interaction between hydrophobic atoms, and, where applicable, hydrogen bonding.
Affinities were computed from AutoDock Vina26. The top two scoring sites from A5UMI1 and Q02394 are in bold. These sites were used to rank the ligands in Table 3.
Statin ligands highlighted in green are lactone form, or red if hydroxyacid form. F420 ligands are in blue. Tautomeric representations are included in each average. Standardized ligands are prefixed with “STD_,” those without standardization are prefixed with “RAW_” (see text). Ligand names have suffixes containing either the PDB entry they were originally extracted from, or their respective PubChem29 CIDs.
Given the rank ordering in Table 3, several observations became evident:
1) Consistent with Sharma et al.14, the lactone form statins docked into each site with favorable site interactions (i.e. lower docking scores) as compared to F420 for the same sequence/site grouping.
2) The statin lactone forms generally had more favorable docking scores, even relative to the native template PDB ligands.
3) The statin hydroxyacid forms had less favorable docking scores and typically scored in the middle with some of the F420 forms.
4) The F420 scores were generally the lowest for each sequence/site models of A5UM1 and Q02394.
Table 4 (a,b) details the AutoDock Vina scoring metrics of lovastatin-lactone v. lovastatin-hydroxyacid across the top two modeled sites. The lovastatin lactone form had better AutoDock scores across each site as compared to the hydroxyacid form. Similarly, the calculated affinity (kcal/mol) of the lactone form was better within both modeled A5UMI1 sites. Figure 2 depicts the best scoring lovastatin-lactone and –hydroxyacid poses in the A5UMI1 modeled site (top) and the Q02394 modeled site (bottom). The A5UMI1 modeled site is more spherically form fitting while the Q02394 modeled site is more elongated. The A5UMI1 site also contains a greater concentration of hydrophilic resides (depicted in cyan in Figure 2). In each modeled site, the best scoring lactone and hydroxyacid form were docked roughly in the same position with similar interactions, however the lactone form contained more favorable intermolecular feature.
AutoDock4.1Score is a weighted sum of steric interactions (Gauss 1, Gauss 2, and steric), repulsion, hydrophobic interaction between hydrophobic atoms, and, where applicable, hydrogen bonding26.
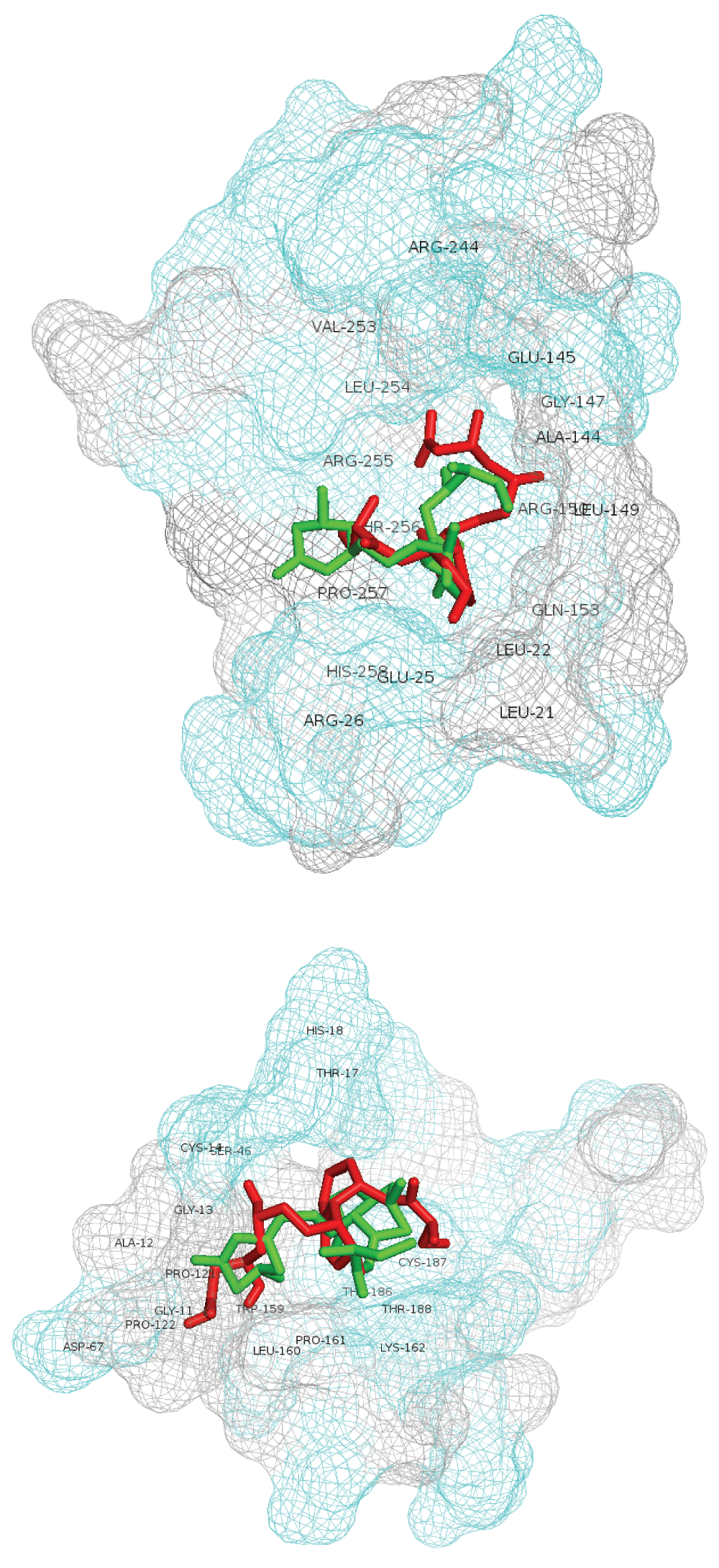
Best scoring lovastatin-lactone and -hydroxyacid poses in A5UMI1 3IQZB_SiteSeeker2 (top) and Q02394 4JJF_SiteSeeker (bottom). Lovastatin-lactone form is shown with green sticks and hydroxyacid form with red sticks. Residues within 5 angstroms of ligands are labeled. Hydrophilic site residues are shown in cyan and hydrophobic residues in gray.
Figure 3 depicts lovastatin-lactone (top) v. F420 (bottom) docked into the top A5UMI1 modeled site (see Dataset 2 helps to visualize and perceive additional detail depicted). Lovastatin-lactone had better AutoDock scores and more favorable calculated affinities – despite having fewer hydrogen bond interactions. Both ligands appear to be interacting with ARG-255, ARG-150, and GLN-153, though F420 seems to also interact with ARG-244. F420’s fit is also considerably more constrained, which explains why its AutoDock Vina score is 4.4× worse than lovastatin-lactone’s score.
Given the large number of ligand-to-site docking scenarios, we were able to observe several key trends that together suggest that statin binding is likely for the two key targets in question A5UMI1 and Q02394. In most cases, the lactone form appears to have preferential binding over the hydroxyacid form and F420. And in many cases, lovastatin/lactone and simvastatin/lactone appear to have preferential binding to even the native ligands found in the PDB templates used to model Q02394 and A5UMI1.
The docking simulations are consistent with those from a recent phase II clinical trial (NCT0249562335) with a proprietary, modified-release lovastatin-lactone (SYN-010) in patients with constipation-predominant, irritable bowel syndrome, which showed a reduction in symptoms and breath methane levels compared to placebo. Given that the lactone form seems to preferentially bind, the next stage of the project is to identify molecules with similar features to lovastatin-lactone that also show similar or better receptor-site interaction potential.
F1000Research: Dataset 1. Raw data for ‘Lovastatin lactone may improve irritable bowel syndrome with constipation (IBS-C) by inhibiting enzymes in the archaeal methanogenesis pathway’, 10.5256/f1000research.8406.d11791736
Figshare: Lovastatin-lactone v. F420 in the A5UMI1 site. doi: 10.6084/m9.figshare.3126538.v137
SM: Finalized analysis workflow, suggested additional hypotheses, performed the experiments, and wrote first and subsequent drafts of the manuscript.
JS: Provided many helpful suggestions during the manuscript preparation.
JK: Made many helpful suggestions during the initial planning and during manuscript review.
MP: Provided insights into methane microbiology and reviewed all drafts.
VW: Co-developed the research question and design of experiments and reviewed all drafts.
KG: Originated the research idea, the major hypotheses and a draft analysis plan and reviewed all manuscript drafts.
All authors approved the final manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 22 Jun 16 |
read | ||
|
Version 2 (revision) 28 Apr 16 |
read | read | |
|
Version 1 08 Apr 16 |
read | ||
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)