Keywords
PARG, PARP, olaparib, DNA damage response, Base excision repair, MMS, ADP ribosylation
PARG, PARP, olaparib, DNA damage response, Base excision repair, MMS, ADP ribosylation
The figure legend of figure 3a and 3b has been corrected and other minor typos. Clarification of the methodology for the detection of nuclear spots has been included.
See the authors' detailed response to the review by Dik C van Gent and Titia Meijer
See the authors' detailed response to the review by Xiaochun Yu
Cells use a varied array of post-translational protein modifications to regulate signalling pathways. One of these is ADP ribosylation whereby single units or multiple, branched polymers of ADP are covalently attached to a target protein. For example, poly(ADP) ribosylation (PARylation) plays a particularly important role in base excision repair with poly(ADP) ribose (PAR) polymerase 1 (PARP1) detecting single strand breaks that occur during this pathway. PARP1, which binds to these single strand breaks undergoes auto-modification creating up to 200 PAR chains1, that subsequently recruit the rest of the repair machinery including XRCC1 and POLB to complete the repair. The ADP ribose chains on PARP are hydrolysed by the enzyme poly(ADP) glycohydrolase (PARG). The correct functioning of this pathway is key for repair to complete. Cancer cells rely on DNA repair more heavily than normal cells and inhibitors of these pathways have been in preclinical and clinical evaluation for a number of years2. The success of this strategy is exemplified by the inhibition of PARPs using olaparib that recently gained regulatory approval for use in ovarian cancers3. However, as auto-modified PARP1 is less able to bind DNA, inhibition of PARG has also been hypothesized as a suitable therapeutic target. This is even more germane as there are now 17 known members of the PARP (otherwise known as ADP ribosyl transferase diptheria-like; ARTD) family yet no known close homologues of PARG. PARG inhibition may therefore offer a more direct approach to derailing the DNA repair pathway without the problems of redundancy. Molecules that are claimed to inhibit PARG have existed for some time. Many of these are large tannin-like molecules such as gallotannin which have been shown to have a number of effects unrelated to PARG inhibition (e.g. anti-oxidant properties4). Other compounds, such as APD-HPD and rhodamine-based PARG inhibitors (RBPIs), have shown good specificity for eukaryotic PARG but are either not cell permeable or have only been tested in biochemical assays5–7. Attempts to discover new synthetic PARG inhibitors have resulted in compounds that also inhibit PARP or have low potency8–10. We therefore carried out a high throughput screen (HTS) directed against human PARG and identified a small number of hits which were carried through to a computational and medicinal chemistry programme11. We were mindful of the need to develop assays to detect cell-permeable inhibitors and the method development is contained herein.
Unless otherwise stated, all reagents were purchased from Sigma-Aldrich (Dorset, UK). Methylmethanesulfonate (MMS) was diluted in dimethyl sulfoxide (DMSO) to 250 mg/mL from the purchased stock. Temozolomide was dissolved in DMSO at 20 mg/mL. All cells were purchased from ATCC (LGC, Teddington, UK) unless otherwise stated and regularly checked for mycoplasma and were regularly sent for authentication. HeLa cells were maintained in RPMI 1640 (Sigma R0883) + 1% Glutamax + 10% FBS. PARG KD cells were purchased from Tebu-bio (PARG Hela Silencix 01-00085, Peterborough, UK) and maintained in DMEM + 1% Glutamax + 10% FBS + 125 µg/mL hygromycin B (#10687010; ThermoFisher, Northumberland, UK). All cells were maintained at sub-confluence at 37°C in a humidified incubator containing 5% CO2 in the absence of antibiotics. Mouse embryonic fibroblasts were cultured in DMEM (# 10938-025; Invitrogen, Paisley, UK) + 10% FBS + 1% L-glutamine and H1048 cells were grown in RPMI (# 21875-034; Invitrogen) + 10% FBS. SW620 cells were cultured in DMEM (#D6546) + 10% FBS + 1% L-glutamine. Dose response curves were generated using Prism v5.2 (Graphpad Software Inc, La Jolla, USA).
Exponentially growing HeLa cells were trypsinized and resuspended in complete media before being filtered through a 40 µM cell strainer (#352340, BD Falcon, Oxford, UK). Cells were then counted using a Muse cell counter (Merck Millipore, Hertfordshire, UK) and seeded in 30 µL of media at 4×104 cells/mL in Greiner 384-well plates (#781091, Greiner Bio-One, Stonehouse, UK) and placed in a cell culture incubator. After 16–24 h the plates were centrifuged briefly at 164×g and the cells dosed with compound(s) or vehicle (DMSO) control using an Echo 550 (Labcyte, Dublin, Ireland). Initially an 8-point dose response with two replicates per point was used with doubling dilutions (0.02–30 µM) and this was extended to a 10-point dose response with 3-fold dilutions (0.001–30 µM) as more potent compounds were identified. After 1 h the plate was re-spun and cells co-dosed with different concentrations of MMS (50–250 µg/mL final concentration) or DMSO using the Echo 550 and incubated for a given time (30 min–2 h) at 37°C in a cell culture incubator. Media was removed from the plate by inversion and cells were fixed with ice-cold 95% methanol/ phosphate buffered saline (PBS) for 15 min at -20°C and then washed once with PBS at room temperature. Cells were then permeabilized using PBS/Triton 0.1% for 20 min, and washed once in PBS before adding anti-PAR antibody (10H (#AM80), Merck Millipore) at 1:4000 in antibody blocking buffer (ADB; 5% Fetal bovine serum, 0.1% Tween20 in PBS) and incubated overnight at 4°C. Cells were then washed three times with PBS, before adding rabbit anti-mouse Alexofluor 488 (A11029, ThermoFisher) at 1:1000 and Hoechst 33342 (at 1:5000) in ADB and incubated for 1 h at room temperature and protected from light. Following three washes with PBS, the plates were sealed and images captured using a 10× objective on a CellInsight (ThermoFisher) and analysed using Cellomics Scan compartmental analysis software (ThermoFisher). A threshold determined by assessing the signal in DMSO treated cells was applied to the pixel intensity and a Box Detection application was used to detect objects smaller than five pixels in radius within the nucleus. The mean of the intensity of these nuclear spots at 488 nM or the mean intensity of total nuclear signal at 488 nM was reported. Initial assays shown in the Supplementary data used only a single dose of MMS for 0–60 min. Studies using temozolomide used the same procedure as with MMS, with a stock solution of temolozomide made at 20 mg/mL in DMSO.
Cells in 96-well plates were fixed with the addition of 100 µL ice cold 10% trichloroacetic acid to the media. After 1 h at 4°C, the cells were washed twice with PBS and left to dry. Once dry, 100 µL 0.2% sulforhodamine B (SRB) was add to each well and incubated for 15 min at room temperature. The cells were washed three times with 200 µL 1% acetic acid and then dried. To solubilise the remaining SRB, 200 µL 10 mM Tris pH10.5 was added to each well and the plate incubated with agitation for 10 min. Absorbance at 520 nM was measured on a plate reader (Biotek, Swindon, UK).
PARylation is principally driven by PARPs 1–3 after DNA damage and alkylating agents are known to induce base excision repair (BER) pathways, intermediates of which lead to activation of PARPs12. Our preliminary data showed Hela cells that have been stably knocked down (KD) for PARG were more sensitive to growth inhibition by the alkylating agent MMS (Supplemental Figure 1a). This led to the initial finding that 250 µg/mL MMS induced PAR chains in PARG KD cells and the peak of PAR chains detected was approximately 20 min after MMS addition (Supplemental Figures 1b–d).
Using the same antibody, an immunofluorescence assay was designed to detect PAR chains in cells. Hela cells were used as they showed increased PAR by western blot after MMS and responded to PARG KD by substantially increasing PAR after MMS (Supplemental Figure 1b). We set up a standard assay based on our previous experience and online protocols for nuclear antigen detection. This used 95% methanol/PBS for fixation and 0.1% Triton X-100 for permeabilization. Hela cells were dosed with 250 µg/mL MMS for different amounts of time. Initial analysis of the PAR signal showed an increase in signal at approximately 25 min (Figure 1). A nuclear mask was generated from Hoechst-stained cells to select regions of interest (ROI) in the 488 nm channel (Figure 1 – analysis panels).
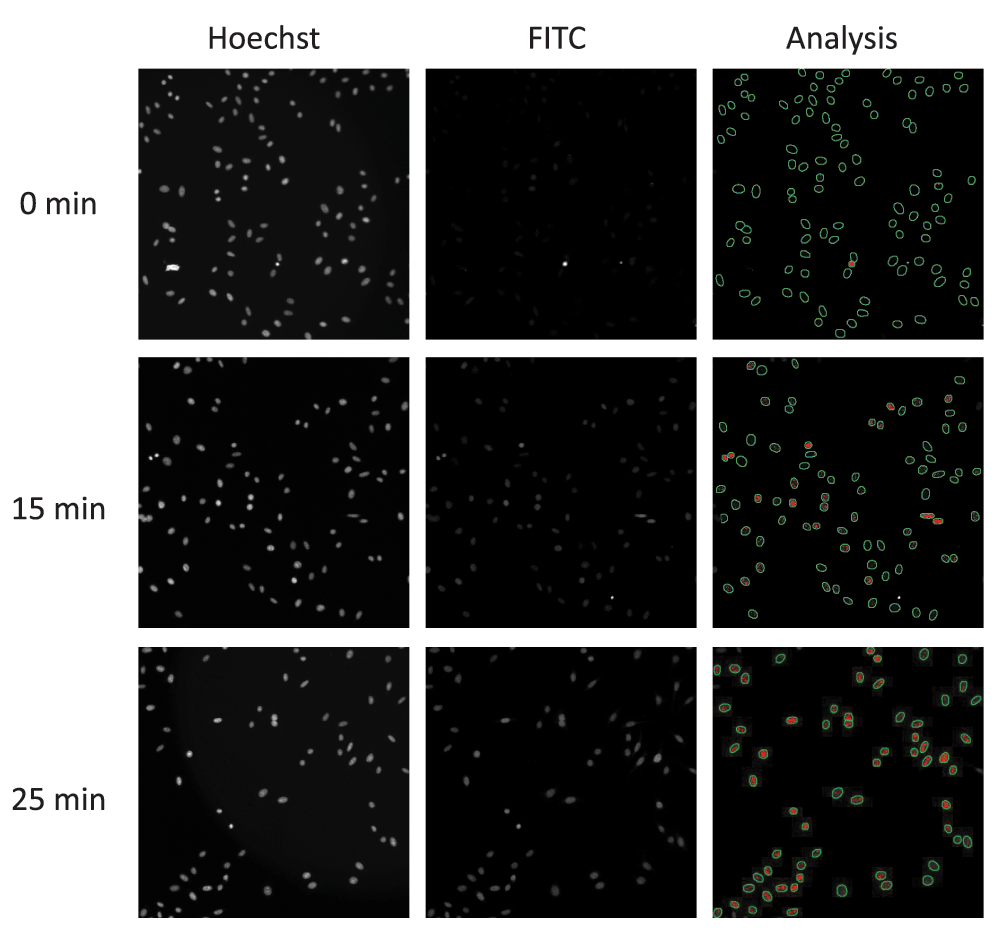
Using a high content imaging system the Hoechst stained nuclei (left-hand panels) are used to create a nuclear mask (green circle in Analysis). Anti-PAR antibody (FITC) detects the increase in PAR chains (centre panels) that is then quantified using the nuclear mask from the Hoechst signal (right-hand panels). Fluorescence intensity is shown as red dots within the nuclear mask.
Different parameters were selected on the Cellomics’ Scan software to report the intensity of the signal within the nuclear area (mask). Both the total intensity of the nuclear PAR signal (Figure 2a; mean_circtotalintensity) and the total intensity of PAR signal points (spots) within the nucleus (Figure 2b; mean_circspottotalintensity) showed a maximum at 25 min and then returned to baseline after 60 min. However, the total intensity of nuclear spots was chosen as the parameter for ongoing experiments as this provided the greatest signal window. We also noted that there was no significant change in cell number over the time course of the experiment (Figure 2c).

(a) The cellular average (from 9 fields) of the total intensity of nuclear fluorescence of PAR after 250 µg/mL MMS as a function of time. (b) The cellular average (from 9 fields) of the total intensity of punctate nuclear fluorescence of PAR after 250 µg/mL MMS as a function of time. (c) Analysis of cell number using Hoechst-stained nuclei after dosing with MMS showing that there is no decrease in total cell number after 1 h treatment.
We initiated a drug discovery programme into PARG inhibitors based on the results of a high throughput screening (HTS) assay of 1.4M compounds11. Using a prototype PARG inhibitor from this programme (PDD00016133) we tested a dose response with 0–250 µg/mL MMS (Figure 3a) and 1 h of incubation post MMS dosing. This time point was chosen because at this time, in the absence of PARG inhibition, PAR chain detection has returned to base level. Pleasingly, DMSO alone (no MMS) had no measureable effect on nuclear PAR chains (Figure 3a). However, PDD00016133 gave a dose-dependent increase in nuclear PAR signal in MMS-treated cells. In our biochemical assay, the same compound gave an EC50 of 0.36 µM (n=22) and we were surprised that the apparent cellular EC50 2.2 µM was significantly less potent. We therefore tested lower concentrations of MMS and showed that decreasing MMS to 50 µg/mL increased the sensitivity of the assay and indicated that further dilutions of the compound needed to be made to generate a full EC50 curve (Figure 3a).

(a) Decreasing the concentration of MMS moved the PARG inhibitor IC50 to the left indicating a greater sensitivity. (b) PAR signal response with 25 µg/mL MMS shows that lower doses of MMS only elicit a nuclear PAR response with longer incubation times. (c) Increasing the time of incubation with 50 µg/mL MMS shifts the PARG inhibitor IC50 curve to the right decreasing sensitivity. (d) A selection of eight PARG inhibitor compounds from a PARG biochemical screen with a range of potencies also shows a range of sensitivities with this PAR chain assay. Different chemical cores of the compounds are shown (green, orange, blue). The compounds are ordered by sensitivity (cmpd 1, least sensitive; cmpd 8, most sensitive). Compound 4 is PDD00016133.
Decreasing the concentration of alkylating agent clearly changed the observed PAR chain response although too little MMS decreased sensitivity (Figure 3b). We therefore investigated how the PAR chain signal changed with time after dosing with 50 µg/mL MMS (Figure 3c). Two hours of exposure to 50 µg/mL MMS provided a dose-response to PARG inhibition with PDD00016133, but with EC50 values increased (6.7 µM) when compared to high doses of MMS seen in Figure 3a. Decreasing the incubation time with 50 µg/mL MMS moved the dose response curve to the left with 30–60 min showing the best response (EC50 = 0.3 µM and 0.5 µM respectively). However, in both of these shorter incubation times we still observed high levels of nuclear PAR signal at the lowest dose of the PARG inhibitor. We therefore increased the dose range and tested a 10-point dose response with 3-fold dilutions between each point. A 1 h incubation time was chosen as this provided optimum sensitivity as well as enough time to dose and process a large number of plates. These assay conditions were tested with a selection of PARG inhibitors with different sensitivities from our biochemical assay. The combination of a 10-point dose response of the PARG inhibitor with 50 µg/mL MMS for 1 h clearly demonstrated that we had cell permeable inhibitors of PARG that ranged from low nanomolar to micromolar potencies (Figure 3d).
We then explored whether other cell lines or other DNA damaging agents could be used with this assay. Firstly, we explored if murine cells responded to MMS. Murine embryonic fibroblasts (MEFs) and the human small cell lung cancer cell line H1048 were dosed with MMS and showed a similar IC50 compared with Hela cells (14.5 µM and 9.0 µM, Figure 4a, b). The PAR chain assay was run on MEFs with the inhibitor PDD00016133 and 50 µg/mL MMS for 1 h. In the absence of MMS there was no increase in nuclear PAR chains detected with this inhibitor. However, in the presence of MMS, the PARG inhibitor led to a dose-dependent increase in nuclear PAR chain signal (Figure 4c). This dose-dependent increase in PAR chain signal after MMS was also seen in H1048 cells (Figure 4d).
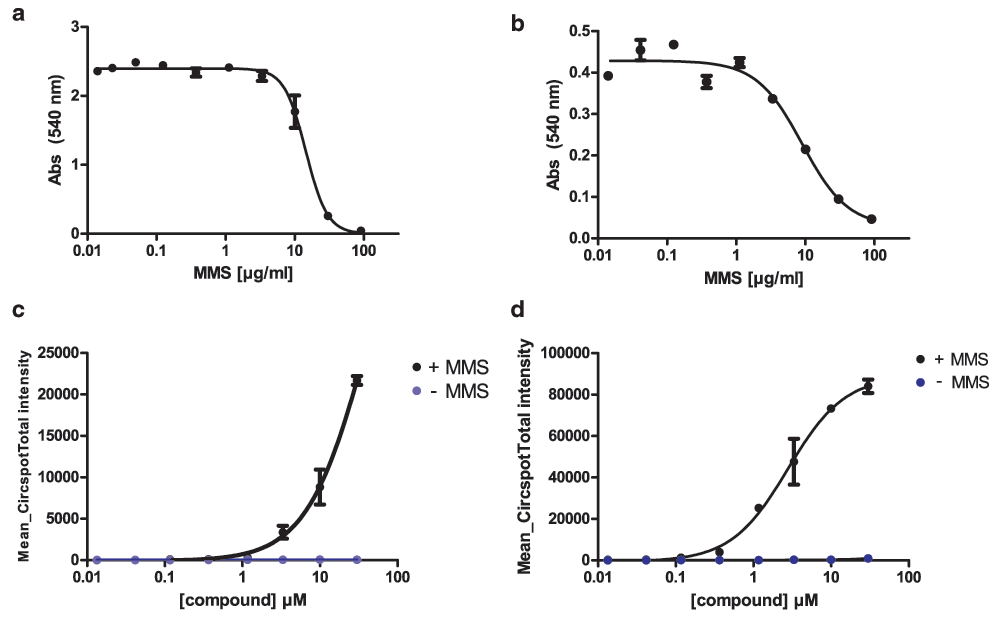
(a) MEFs and (b) SCLC H1048 treated with MMS were fixed and stained with sulforhodamine B (SRB) after 72 h. (c) MEFs and (d) H1048 cells show a dose-dependent increase in PAR chains after MMS treatment.
We next explored whether a more clinically relevant DNA alkylating agent could induce PAR chains. Temozolomide (TMZ) is a DNA alkylating agent and is used as a standard-of-care treatment for patients with glioblastoma13. Here we used the colorectal cancer cell line SW620 that we knew was sensitive to alkylating agents (Figure 5a) and which has been used in xenograft studies in combination with TMZ and the PARP inhibitors olaparib or AG01469914,15. First we used the same assay conditions to determine whether increasing concentrations of TMZ induced PAR chains that could be maintained by inhibiting PARG with a potent inhibitor (compound 8 from Figure 3d). As expected from the previous cell lines, one hour after treatment with TMZ alone there was no PAR signal detectable in SW620 cells. However, the presence of 300 nM compound 8 led to a TMZ dose-dependent increase in PAR signal (Figure 5b). Furthermore, using a set amount of TMZ (150 µg/mL) we were able to show that PARG inhibition by compound 8 led to a dose-dependent increase in PAR signal (Figure 5c). Unsurprisingly, pre-treatment with olaparib, which prevents PARP1 PARylation did not lead to any increase in PAR signal after TMZ treatment.

(a) SW620 cells treated with MMS for 72 h and stained with Hoechst show a similar dose-dependent decrease in proliferation in comparison with other cell lines tested (b) SW620 cells pre-treated with compound 8 at 300 nM increase PAR chains in response to 1 h temozolomide (1.5 µg/mL–200 µg/mL). However, pre-treatment with DMSO or olaparib (300 nM) had no effect on PAR chains at this time point. (c) SW620 cells treated with increasing concentrations of a PARG inhibitor (cmpd 8) and 150 µg/mL temozolomide for 1 h showed a dose-dependent increase in PAR chains. As expected at this time point treatment with olaparib had no effect on PAR chains.
Finally, we quantified the relationship between individual assay results in Hela cells for PDD00016133 against its geomean over a period of 2½ years (Figure 6). Over 100 assays with PDD00016133 were run during that time, of which 85% were within ±0.25×pIC50 of its geomean. Interestingly, cell cultures that had passage numbers of less than 8 or more than 19 were more likely to give results for this compound that exceeded these limits.
A number of molecules have been used to inhibit PARG but concerns have been raised as to their selectivity and potency both in biochemical assays and in cells. As part of a drug discovery programme for PARG inhibitors we designed and optimized a cell assay for PARG inhibitor activity. Our initial work showed that the higher dose of MMS (250 µg/mL) resulted in a complete dose response curve for our PARG inhibitor but potency was lower than we expected. By reducing the amount of DNA damage the sensitivity of the assay increased, presumably as the detection of SSBs by PARP1 and its associated machinery was not overwhelmed. However, the lowest dose of the PARG inhibitor still resulted in relatively high levels of PAR chains after 30–60 min that was resolved when the dose response was extended.
Immunofluorescence assays using the 10H mouse hybridoma antibody for detecting PAR were first published over 20 years ago16. However, detailed quantification using immunofluorescence of the amount of PAR chains found after DNA damage appears to be absent from the literature. Instead, enzyme linked immune absorbance assays (ELISA) or dot-blots have been used to detect the reduction of PAR chains following the use of PARP inhibitors17–19. There have been studies that have followed the kinetics of PAR chain accumulation after treatment with the alkylating agent MNNG or the oxidant H2O220 but none on the increase of PAR following temozolomide treatment. However, studies using RNA interference have been able to show a delay in hydrolysis of nuclear PAR after treatment with H2O2 and knockdown of PARG21.
The suitability of this assay for screening PARG inhibitors in Hela cells is clear from the data collected over time and with different compounds (Figure 3d and Figure 6). However, MEFs and H1048 cells displayed a response that was indicative of the Hela cell response prior to optimisation (Figure 4c, d), suggesting that more method development would be needed if these cells were going to be used for routine testing.
This assay was designed to test for PARG inhibition after a DNA damage signal. However, a number of PARPs are involved in non-DNA damage related processes (e.g. tankyrases, reviewed in 22) that take place outside the nucleus. Hydrolysis of PAR chains created by other PARPs is likely to involve PARG or ARH323. It is possible that these PARG inhibitors prevent such processes but modification of this assay would have to be undertaken to detect non-nuclear PAR.
In summary, we have designed a sensitive assay to test for PARG inhibition in cells. The assay was appropriate and stable for long term use and detected PAR chains from different species and different cell lines.
F1000Research: Dataset 1. Raw data for Figure 2–Figure 6 in ‘An assay to measure poly(ADP ribose) glycohydrolase (PARG) activity in cells’, 10.5256/f1000research.8463.d11922524
D.J., S.D., N.H., E.F., L.G., P.K., K.S. and K.E. designed and conducted the biological experiments. D.J. and S.D. conceptualized the experiments and I.W., M. O’C and D.O. provided strategic direction. D.J. prepared the manuscript.
There are no competing financial interests to declare. Stephen Durant, Kay Eckersley, Kerry Shea, and Mark O’Connor were all employees of AstraZeneca PLC at the time experiments took place.
This work was funded by Cancer Research UK (Grant numbers C480/A1141 and C5759/A17098).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figure 1. Preliminary results during assay development. (a) PARG KD Hela cells (PARG Silencix) are more sensitive to MMS than control cells (IC50 6.2 µg/mL vs 16.5 µg/mL) over 5 days. (b) PAR detection by western blot shows that MMS induced PAR response in control (-) and a substantial response in PARG KD cells (KD). (c) HT29 cells treated with 250 µg/mL MMS show an initial increase in PAR signal at 20 min with a gradual reduction in detectable PAR chains. (d) Cartoon of proposed PAR chain response in cells.
Click here to access the data.
Supplementary methods and notes on compounds used.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 15 Sep 16 |
||
|
Version 1 25 Apr 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)