Keywords
Optogenetics, locomotor activity, Serotonin (5-HT), Glutamate, GABA, acetylcholine
Optogenetics, locomotor activity, Serotonin (5-HT), Glutamate, GABA, acetylcholine
Controlling the activity in neural circuits, while monitoring the effects on acute and chronic behaviors, is a means of addressing the function of defined neural pathways. This concept is related to the more common pharmacological approaches but in some cases with less precision to accomplish some of the same tasks. To selectively manipulate a subset of neurons within an easy organism to rear and maintain, Drosophila combined with optogenetics is ideal. The animal model and exercises provided allows for a ‘hands on’ inquiry-based learning module for high school and college courses, which emphasize life science topics. The current article presents a teaching module that is designed to integrate modern genetics, engineering, physics, life sciences, modeling and experimental design for use with high school and college students. Researching the primary scientific literature and utilizing the related findings, as well as postulating the outcome for newly designed experiments based on the results one collects, allows students to test their own predictions and draw hypotheses. This approach provides autonomous learning among student groups. The measureable outcomes with obtaining quantitative data for analysis and interpretation are valuable learning experiences. Based on one’s findings in the initial experiments, one can readily redesign experimental paradigms to test the formulated hypotheses utilizing one’s own prior data. The integration with Arduino hardware and software opens the doors for students to a world of writing code with an experimental purpose, and independence in experimental design.
The underlying science in the module proposed by the current article focuses on neurobiology. The seminal discoveries by Hubel & Wiesel (1970) demonstrated that activity in sensory input and within the central nervous system (CNS) is indispensable in the development and maintenance of neural circuits. This concept is also essential for development and maintenance of synaptic establishment at the neuromuscular junction (NMJ) of skeletal muscles (Balice-Gordon et al., 1990; Lømo, 2003). In some cases, the activity profile must occur prior to developmental time points before the neural circuits become more hardwired. After such a critical period in synaptic formation, the circuit is not as dependent on activity for competition with other neurons for the establishment of connections. This fundamental phenomenon occurs in organisms from fruit flies to humans. It is known in mice that even after established connections are made in adults, the terminals at NMJs are not fixed to one spot on a muscle fiber: The motor nerve terminals grow out and pull back over time while continuing to communicate with the muscle fibers (Lichtman & Sanes, 2003).
If motor neurons, which are normally innervating a muscle, are removed, then other motor neurons will take control of the target and innervate it. Thus, motor nerves are searching out targets not already committed by other synaptic inputs (Chang & Keshishian, 1996). This was examined in embryonic and larval Drosophila by laser ablating various body wall muscle fibers during development. Even pharmacologically activating or silencing neural circuits during development can have long term consequences in neural connections and overall physiological functions (Smith et al., 2015). For example, exposing rodents to nicotine during development changes the dendritic morphology within the CNS, which lasts into adulthood (McDonald et al., 2005). Even short exposures to nicotine in the juvenile stages have long lasting effects in adults for these mice (Ehlinger et al., 2014). It is also established that collective synchronized synaptic activity is important for development of the neural structure (Winnubst et al., 2015). Thus, long term consequences in the established neural circuitry within the CNS and at the NMJ can occur based on neural activity when the initial circuits are being wired.
A guided self-inquiry based approach to learning science has been demonstrated to be a very effective means for student learning in the long term (Bradforth, et al., 2015; Waldrop, 2015). The engineering design with Arduino systems is a very engaging educational experience, which is sought after in many schools within the USA and abroad (see educational web pages: https://www.adafruit.com/educators; https://www.arduino.cc/en/Main/Education; Bender, 2012; Escudero et al., 2013; Junior et al., 2013; Maxwell & Meeden, 2000; Zalewski et al., 2014). The surge in the use of the Arduino system in high school and college teaching is partly due to the low cost and ease in writing code for operating the system. Students can design experiments with various computer codes to control the duration of light on-off time period and frequency of stimulation to observe how activating or inhibiting a specific set on neurons alter development and behavior of Drosophila larvae or adults. Arduino and associated LED required hardware is relatively inexpensive, <$20 USD for an individual unit; however, making a series of units with one power supply is cheaper for adding additional units. Class sets can be used in subsequent years, so an initial investment has a long term use. There are dozens of demonstration videos on YouTube for a wide variety of inventions and coding using Arduino.
In the educational module presented in this article, we demonstrate an approach with optogenetics to selectively activate the neurons synthesizing the neurotransmitter GABA (glutamate, serotonin, and acetylcholine). The approach used to stimulate these selective neurons is to activate light sensitive channels expressed in these neurons. Different Drosophila lines are used for each type of neurotransmitter. The ability to control the stimulation with light is managed by an Arduino system or a simpler system with a 9 Volt battery and an LED source. Students can readily add single units or build parallel outputs with discrete parameters for controlling the LEDs. Thus, this allows various parameters to be tested simultaneously in the same laboratory setting. Since many of the experimental paradigms presented by the module are novel, many unanswered questions remain to be answered in neurobiology, and students may uncover unique findings worthy of publication in scientific journals.
This educational module is also designed to embrace the Next Generation Science Standards (NGSS Lead States, 2013), through approaches scientists employ in the development of scientific knowledge. NGSS recommends that models be used in Developing, Evaluating, Using, and Revising explanations and predictions of science phenomena. The students will be able to construct models in neural circuits to explain the observed behavioral phenomenon to make sense of what they observe. The direct real life examples concerning how neural circuits develop in one’s self, as well as in other animals, is of general interest, but also has applied implications for medicine and health. The ability to manipulate various neurotransmitter systems and stimulation paradigms promotes experimental design and redesign based on the observed findings from each experiment. This is an integral aspect of the NGSS. The approach presented herein promotes explanations of the findings in order to set a new or altered stimulation paradigm, as the students continue to study a phenomenon in different contexts. In addition, this article discusses some of the techniques used in a trial of this module for sophomore high school students in Louisville (KY, USA), senior high school students in Sommerset (KY, USA) and college level juniors and seniors at the University of Kentucky. (The outcomes of the trial are detailed further in the section Instructor feedback).
Some of the experimental procedures require being able to make selective genetic crosses of two different Drosophila lines. To perform the crosses, it may be necessary to identify male and female adults and to be able to obtain virgin females (Figure 1). The instructors of the module can decide on their resources (dissecting microscopes and time management of students) for either performing the crosses themselves, or if the students should be given the time to make the crosses or be provided an explanation. As a learning experience, the teacher could allow the students to try these procedures, but have a cross already prepared for class use. A number of online resources are available to see the differences in males and female adult flies; the presence of black tuft of hairs on the forelegs indicates a male fly (Figure 1). It is good to compare the flies side by side to tell the differences.
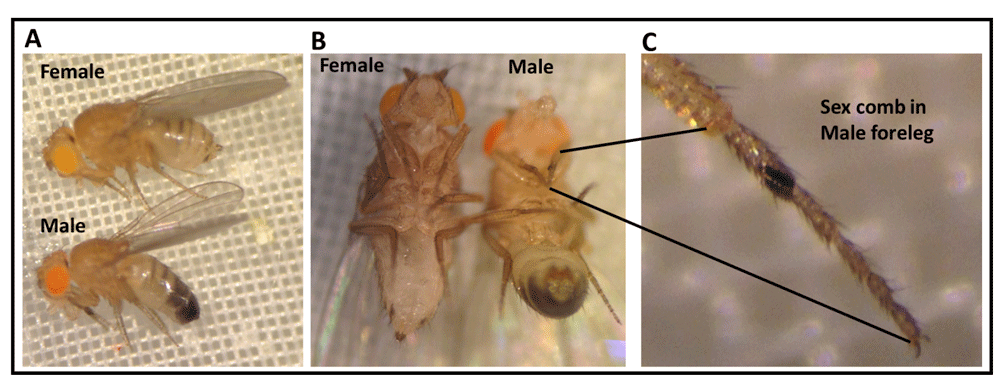
(A) Morphological characteristics and sexual dimorphism of adult Drosophila melanogaster (lateral view). Adult female fly (top) has a light colored abdomen region; however, adult male fly (bottom) has a dark posterior abdomen region. (B) Morphological differences between male and female flies (ventral view). (C) Magnified view of the male fly foreleg shows male specific sex comb structure.
There are some procedures where the fly lines obtained can be directly examined without having to make filial 1 (F1) generations with selective crosses. The line that expresses the light-activated channelrhodopsin-2 in motor neurons is OK371-Gal4;UAS-ChR2H134R-mcherry (homozygous line, there are two copies for each construct). This line is made by crossing w1118;P{GawB}VGlutOK371 (Bloomington Drosophila Stock Center at Indiana University (BDSC); catalog no., 26160) with w*; P{UAS-H134R-ChR2}2 (BDSC; catalog no., 28995; Pulver et al., 2011). When trialing this module, we used another recently created ChR2 line, which is very sensitive to light, called y1 w1118; PBac{UAS-ChR2.XXL}VK00018 (BDSC; catalog no., 58374; Dawydow et al., 2014). Virgin females from w*; P{UAS-H134R-ChR2}2 were crossed with males of D42-Gal4 (BDSC; catalog no., 8816) for also being expressed in motor neurons. Trh-Gal4 (BDSC; catalog no., 38389), Gad1-Gal4 (BDSC; catalog no., 51630), or ppk-Gal4 (BDSC; catalog no., 32078) to express ChR2-XXL variant in serotonergic neurons, GABAergic neurons or type IV sensory neurons, respectively. In the trial module, we also used UAS-H134R-ChR2;Trh-Gal4 (III) homozygous line, which was kindly provided by Dr. Andreas Schoofs (University of Bonn Life & Medical Sciences Institute (LIMES), Bonn, Germany; Schoofs et al., 2014), to compare behavioral effects with the more light sensitive ChR2 line. Table 1 outlines which crosses of flies can be used from these lines mentioned above for targeting the desired neuronal subtypes.
w*; P{UAS-H134R-ChR2}2; Trh-Gal4 (homozygous line) ChR2 expressed in serotonergic neurons.
There is no need to make crosses as this line is homozygous. The larvae or adults should be raised on food supplemented with all trans retinal (ATR), which is a cofactor essential for ChR2 function, since unlike mammals the flies cannot synthesize sufficient amount of ATR for ChR2 function) and a control group without ATR (use ethanol (EtOH) as a vehicle since ATR is dissolved inside absolute ethanol).
All-trans-retinal is a cofactor for the channel rhodopsin which increases the sensitivity to light and increases single channel conductance (Dawydow et al., 2014). ATR (500mg; available from Sigma-Aldrich, St. Louis, MO, USA) is dissolved in 17.6 ml absolute ethanol to make 100mM stock solutions. Then, 100µl of 100mM stock solution is transferred to small tubes, wrapped with aluminum foil and kept in a -20°C freezer. The ATR should be kept away from light, since it is light sensitive; it would be degraded and become ineffective if it is exposed to light for a long time.
Preparation of fly food supplemented with ATR. In order to prepare fly food supplemented with 1mM ATR, 10ml fly food is dissolved in the microwave. The food is left to cool, then 100µl of 100mM ATR is mixed well with the fly food, or 100µl of absolute ethanol is mixed with food as a control. The food vial should be wrapped in aluminum foil and the food left until well solidified (flies may stick to wet food). The larvae or adult flies for whichever experimental lines to be tested are then transferred from their vial to an ATR-food-containing vial and are kept in a dark place (to keep the ATR from degradation) at room temperature (22-23°C).
Locomotion behavior of larvae is assessed by placing a single larva on an apple-juice agar plate. The larva is left for one minute to acclimate to the new environment. Having the room lights off or very dim while the students work might be difficult to achieve in some classrooms. The body wall contractions (BWCs) are counted for one minute (BWCs/min) while the larva is exposed to regular white light. Then the body wall contractions are counted for one minute while the larvae is exposed to blue light (470nm wavelength; a dispersed-soda-can device can be used, see Figure 2). Also, body wall contractions are counted while the larva is exposed to focused focal blue light (a focused light through a microscope eyepiece with a mounted LED can be used, see Figure 2). This assay can be performed for first, second and third instar larva. In the module trial, the typical behaviors of third instar larvae are shown in Figure 3 for flies fed and not fed ATR, as well as for dim white light, diffuse blue light delivered by a soda can set up and a focused blue light with a microscope eyepiece objective. Notice in Figure 3B3 the contracted larvae. The microscope eyepiece can be bought on Amazon.com as 10X eyepieces; a wide base type is most useful, so the LED can fit inside. Table 2 provides a template in which students can record the type of behaviors observed with this experimental paradigm.
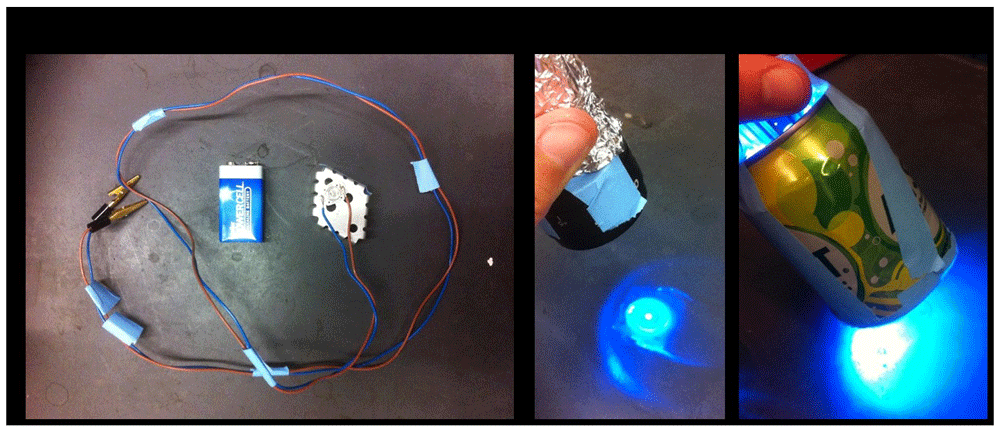
(A) A blue light emitting diode (LED; wavelength = 470nm) is glued on a cooler plate with a temperature resistant glue. The LED light is connected to a 9V battery. Various intensities of LED light can be used by attaching the LED to (B) a microscope ocular lens (x10), which gives off high intensity light or (C) a soda can with the bottom removed and the LED placed though the top, which gives a low intensity diffuse light.

OK371-Gal4 (Gal4 driver specific for motor neurons) is crossed with UAS-ChR2H134R-mcherry Drosophila line (this line is homozygous foe both Gal4 and UAS constructs). The progeny expresses ChR2 in motor neurons. (A) The larvae were raised in fly food, which was not supplemented with all-trans-retinal (ATR), a cofactor important for ChR2 membrane integration and function. (A1) The body wall contractions (BWCs) are counted on an apple juice agar plate for 1min when the larva is exposed to regular light. (A2) The larvae is exposed to low intensity blue LED light (470nm) for 1min while the BWCs are counted. (A3) The crawling behavior of larva is observed when it is exposed to intense blue light for 1min. (B) The larva was fed ATR (1mM), which was mixed with fly food. The body wall contractions are counted when the lava is being exposed to regular light (B1), low intensity blue light (B2), or high intensity blue light (B3). The larva does not respond to the low intensity light although when it is being exposed to high intensity blue light, the BWCs contract, which can be observed by shortened body length (B3).
Rolling behavior in larvae. Assessing rolling behavior is performed by placing a single larva on the surface of an apple-juice agar plate. The occurrence of rolling behavior can be counted for the 1st and 2nd minute. The percentage of larvae that show rolling behavior can be presented in graphical form, as shown in Figure 4 (module trial results), for a ChR2 being expressed in type IV sensory neurons in third instar larvae and stimulated with blue light. The fly lines crossed for this experiment are the UAS-ChR2-XXL and ppk-Gal4 and the food was without ATR or supplemented with ATR (1mM). A sample size of 20 larvae were tested for this data set
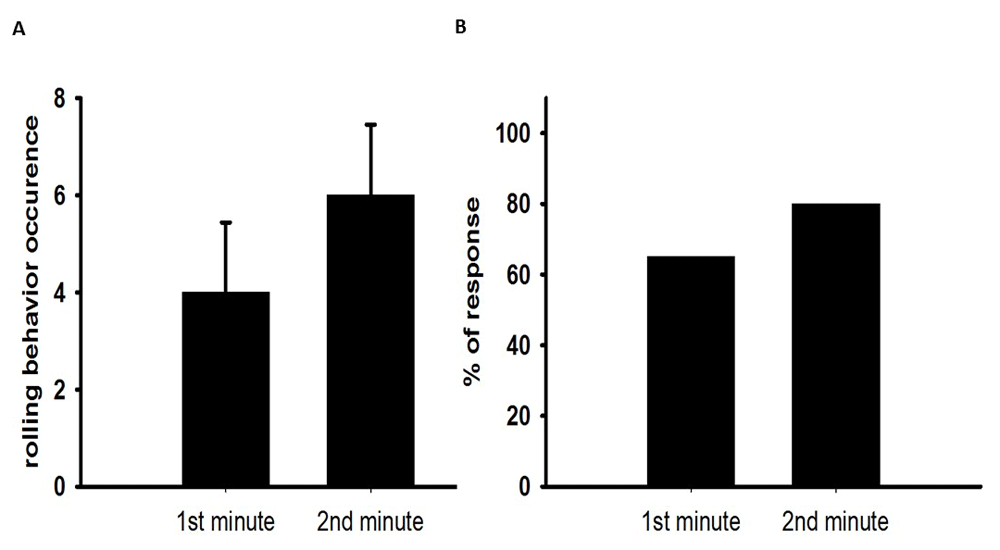
(A) Shows the occurrence of rolling behavior in the 1st and 2nd minute of light exposure (normal scope light). (B) Most of the larvae showed rolling behavior when they are exposed to light.
The participants can fill in a data table such as this one presented.
For adult behaviors, left over larvae from conducting the larval behaviors can be used, the 1st crosses should be saved and grown to adults. Thus, the differences between the larval and the adult lines can be compared with the same crosses. Also, if ATR-tainted food from the larval assays is saved, this can be used to feed the adults. The adults should be a few days old before conducting the following behavioral experiments to insure they have built back up the levels of ATR in the body. There are a number of behavioral assays that are commonly used for adult Drosophila (Badre & Cooper, 2008; Nichols et al., 2012; http://www.sdbonline.org/sites/fly/aimain/6behavior.htm). For some of the assays, the separation of males and females should be considered, as there are differences in the size and weight of the adult flies. Also, as the adults age there may be differences in their behaviors.
The two commonly used behavioral assays that are relatively easy to implement, but informative for the biological concepts, are the negative geotaxic and phototaxic assays, which are described below. These assays can be expanded on for deeper investigation into the neurobiology of the flies. Also, these behavioral assays allow for data gathering, redesign and vivid discussion for inquiry based labs.
Negative geotaxic assay. Adult flies aged 2–8 days can be anesthetized with CO2. Males and females are sorted and transferred into separate vials, containing food, in cohorts of 10–14 flies. The flies should be left to recover for 24h before running the experiments. A plastic vial (Drosophila culture cylindrical vial 1-1/4" diameter x 4" tall; http://www.enasco.com/product/SB11136M) can be marked at 8cm length, and the 10–14 fly cohort transferred to an empty marked vial. Another plastic vial can be placed on top of the marked one (Figure 5). The flies should be left for one minute. The vials can be tapped on a table to knock down the flies to the bottom of the tube. Then the number of flies that climb across the 8cm mark within 10sec can be recorded, as shown in Figure 5. This procedure can be repeated a few times with tapping to knock the flies down to the bottom of the vial each time. Table 3 provides a template in which students can record the data for this assay of adult behaviors.
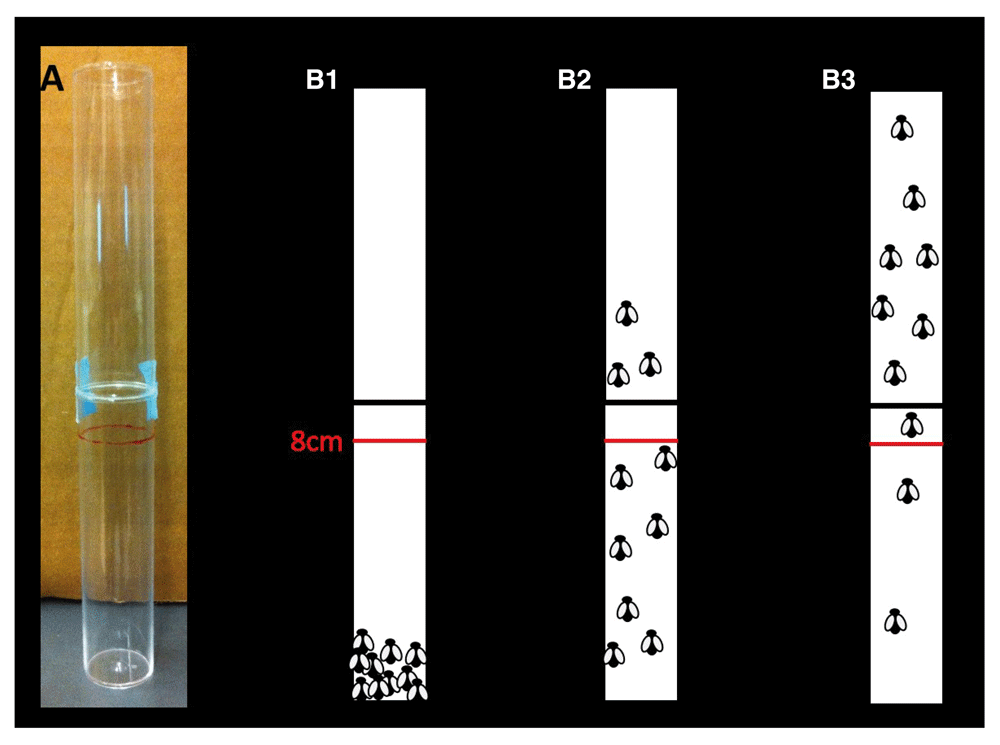
(A) Two plastic tubes are put together for this assay. 10–14 adult male or females flies are transferred to an empty plastic tube which is marked at 8cm length. Second plastic tube is put on the top of the first marked plastic tube and sealed with tape. (B1–B3) The tube is tapped until all the flies fall into the bottom of the first tube (B1). The flies start climbing up on the wall of the plastic tubes. After 10 seconds the number of the flies that cross the 8cm red line is counted which shows the percentage of the flies that are crossed the line in 10sec. (B1) 0%, (B2) 30%, (B3) 80% of the flies are crossed the red line.
During the module trial, we used flies that were expressing ChR2 in motor neurons and fed ATR 1mM (UAS-CHR2H134R-mcherry;OK371-Gal4). This particular line (ChR2H134R) is a strain where the protein (the channel rhodopsin) has been altered with different amino acids and is not as sensitive as the ChR-XXL line. This line did not show a large difference from the 1st min of recovery time after the blue light was turned off to the 3rd minute of recovery time for the percent number of flies passing the 8 cm mark. The bars that are labeled ‘crawl’ represents the flies that are crawling around the bottom of the vial (Figure 6). Using the very sensitive strain of flies (ChR2-XXL), where the channel demonstrates high sensitivity to blue light, the percentage of flies recovering took much longer than for the UAS-CHR2H134R-mcherry;OK371-Gal4 cross (Figure 7). Also, the UAS-ChR2-XXL/+;D42-Gal4/+ cross targeted motor neurons, which express D42. The recovery time of paralyzed flies for this fly strain was not even fully recovered after 14 minutes after the blue light exposure.

These flies are expressing ChR2 in motor neurons and also are fed ATR 1mM (UAS-CHR2H134R-mcherry;OK371-Gal4). The blue light does not exert influence on the negative geotaxic assay since the blue light cannot penetrate well the thick dark adult cuticle.

(A) The crawling and negative geotaxic behavior of adult female flies is decreased after 25 second blue light (low intensity) exposure. After 14 minutes the flies restored their normal climbing ability. (B) The ability to crawl and climb was markedly compromised in adult male flies being exposed to blue light for 25 seconds. The crawling ability restored after 6min of paralysis although the climbing behavior went back to normal after 12 minutes of paralysis. These flies were raised of food supplemented with ATR 1mM.
Extra details on this behavioral assay is found in Ali et al. (2011). When using a similar assay, one can also measure the percent of flies which start to crawl as an index. This can be tried with motor neurons drivers or other types of neuronal drivers. In the module trial, we used a subset of sensory neurons, referred to as Type VI sensory or pickpocket neurons, which had ChR2-XXL expressed (Figure 8). It is obvious in the first experiment (by observing how many flies crawled or moved up the tube) that it was difficult for the flies to walk up the walls of the tube, but in subsequent experiments more were able to walk up the tube.

After 5 sec blue light exposure, some of the flies were paralyzed for 1–2 seconds then they recovered well. As it is shown that the first trial (T1) after blue light exposure, the flies do well in climbing assay; although, in the second trial (T2 after blue light exposure), the flies climb the middle of the bottom tube then they stop climbing further. They recover quickly in the following trials.
Phototaxic assay. To conduct this assay, a device with a 25cm long plastic tube and light source at one end in a dimly lit room is used to assess the phototaxic behavior of the adult flies. The tube is narrow enough not to allow the adults to fly, but only walk up the tube. Also a standard small LED maglight fits snuggly in one end (Figure 9). The male or female flies can be anesthetized by a quick exposure to CO2 or by placing a vial in ice for 25–30sec. Individual flies are placed in each apparatus. The flies need to recover for at least 10min. Each apparatus with an individual fly should be positioned vertically and tapped until the fly falls to the bottom of the tube, which is closed by a rubber stopper. The time the fly crosses a 10cm line and a 20cm line can be recorded as a measure. This apparatus could be positioned horizontally or vertically, but vertical placement examines both geotaxic, as well as light sensitivity. A sample table to enter student data is presented as Table 4.

A single male or female fly is transferred into the tube. The tube is tapped until the fly falls into the bottom of the tube. The time that the fly takes to reach 10cm and 20cm is recorded.
The results from the various experiments can be tabulated or graphed in various ways, depending on the variables the instructor and students wish to investigate. Data that can be plotted over time, such as time for the adults to cross the 10 and 20 cm line, can be graphed using free web based graphing software Joinpoint, (http://joinpoint.software.informer.com/), which allows students to work at home or at school. Also, graphing the values for the different experimental lines of flies allows for discussion of the data in relation to biological significance. For high school teachers focusing on The Next Generation Science Standards (NGSS Lead States, 2013), or college instructors wanting more of an inquiry based experience in real life topics for students, the exercises provided can be varied or expanded.
For example, different instar stages can be compared for a particular strain. In the module trial, we used the ChR2 channels in motor neurons with a less sensitive strain (UAS-ChR2H134R-mcherry;OK371-Gal4) which showed different responses between the various instars and measured body wall contractions for one minute (BWCs/min). These larvae were fed ATR 1mM (Figure 10). Also central neurons that utilize different neurotransmitters can be examined for changes in larval, as well as adult, behaviors. We used a line that results in activation of the GABAergic neurons and measured locomotor activity in third instar larvae fed ATR (UAS-ChR2-XXL;Gad1-Gal4). The blue light stimulation resulted in a substantial decrease in body wall movements (Figure 11). This same line can be used for measuring adult behaviors and measuring climbing. A sample of such responses is shown in Figure 12. The adults were fed ATR (1mM) and they showed a reduced ability to climb.
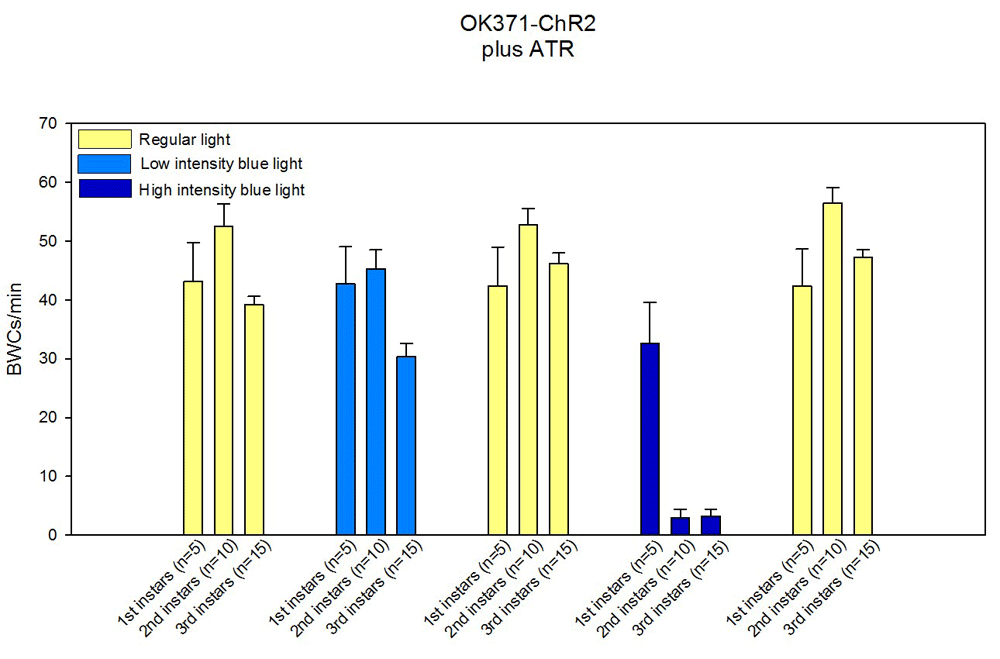
The body wall contractions for one minute (BWCs/min) are being counted while the larva is being exposed to regular light, low intensity blue light or high intensity blue light. The data shows that the first instar larvae do not respond well to even high intensity blue light.

(A) The locomotor activity in larvae fed ethanol (vehicle) does not change with either normal light or blue light exposure. (B) Although, when the larvae which were raised on food supplemented with ATR 1mM and exposed to white light, the locomotor activity significantly decreased. During the exposure to light the larvae first started to contract which was followed by body muscle relaxation.
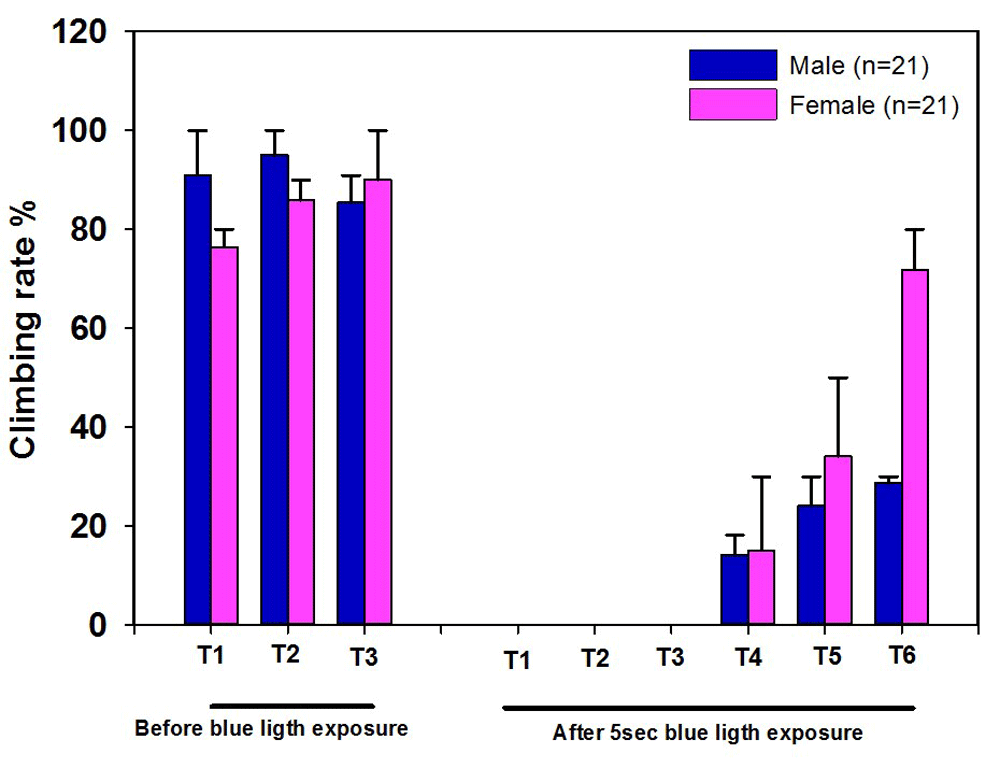
The climbing ability was measured in three different trials before the blue light exposure. After 5sec blue light exposure, the climbing ability was performed in six different trials by knocking the flies down to the bottom of the vial without blue light exposure and measuring how many could crawl. ChR2-XXL activation significantly reduced climbing ability. Flies were fed food supplemented with ATR 1mM. The climbing assay was carried out a dim light room since the bright light might also activate ChR2 channels which make it difficult to perform the assay in a well-lighted classroom.
Instructors can also use published literature and standard textbooks to explain to students that the locomotor behaviors are driven by motor neurons, which activate body wall muscles (Marieb & Hoehn, 2013; Sherwood, 2001). In addition, an instructor can use the illustrations in Figure 13 to examine how the electrical responses are monitored in the muscles and the effect of stimulating various types of neurons. In addition, there are published studies and figures readily found on the web that highlight the various neuronal types within the CNS and ventral cord of larval, as well as adult, Drosophila, which contain different types of transmitters, such as serotonin (as shown in Figure 14). In the module trial, we have shown the effect of activating serotonin producing neurons on behaviors for body wall movements of larvae with a line less sensitive to blue light (Figure 14B; UAS-mCherry-ChR2 H134R; Trh-GAL4, homozygous for both constructs), as well as a line very sensitive to blue light (Figure 15; UAS-ChR2-XXL;Trh-Gal4). The two levels of blue light sensitive lines can also be examined as adults, as we demonstrate in Figure 16.

(A) The nervous system in Drosophila melanogaster third instar larvae expression green fluorescent protein (GFP) in the whole nervous system. (B) Dissected third instar larvae shows m6 muscle fibers and intracellular microelectrode to record excitatory postsynaptic potentials (EPSPs) while the ChR2 in motor neurons are being activated by blue light exposure. (C1) Shows the intracellular recording in OK371-ChR2 minus ATR (CNS intact) third instar larvae. The blue light (low intensity) exposure does not produce any postsynaptic responses in muscle fiber M6 since the larvae is not fed ATR, which is a required supplementation for the action of ChR2. (C2) Shows the intracellular recording in OK371-ChR2 plus ATR 1mM (CNS intact) third instar larvae. Blue light (low intensity) exposure produces responses in M6 muscle fiber which is presented as EPSPs. (D1) Intracellular recording from M6 fiber muscle in OK371-ChR2 minus ATR (CNS intact) third instar larva. Blue light (high intensity) exposure does not activate motor neurons. No EPSPs are seen in this trace although the miniature EPSPs are still present. (D2) the evoked response is being recorded in OK371-ChR2 plus ATR 1mM (CNS intact) third instar larvae while it is being exposed to blue light (high intensity).

(A1) Central nervous system in third instar larva. (A2) Serotonergic neurons expressing mcherry fluorescent protein (UAS-mCherry-ChR2 H134R; Trh-GAL4, homozygous for both constructs). (B) Activation of 5-HTergic neurons did not produce a significant effect on locomotor activity in third instar larvae.

To change serotonergic neuron activity, CHR2 were expressed in serotonergic neuron population (UAS-ChR2-XXL;Trh-Gal4). The body wall contractions were counted in third instar larvae fed on food supplemented with ATR 1mM or ethanol (vehicle). When the larvae, which were fed on ATR (1mM) were exposed to blue light (high intensity), the locomotor activity significantly compromised. However, when the larvae fed on a food without ATR supplementation were exposed to blue light (high intensity), the locomotor activity were not affected (as shown in Majeed et al., 2016).

The electrical activity in serotonergic neuros is increased by expressing ChR2. When the adult flies were exposed to blue light (low intensity), the climbing ability significantly reduced. Both flies groups (UAS-ChR2-XXL;Trh-Gal4) which were fed supplemented with ATR (1mM) or ethanol (vehicle) were affected by the blue light exposure. However, blue light did not have effect on the control lines (UAS-ChR2-XXl/+) (As shown in Majeed et al., 2016).
It may be confusing for students to understand that sensory neurons can present a behavior similar to that of stimulating motor neurons. Instructors should help students to understand neural circuits and that activating sensory neurons can lead to motor neuron activation. The activation of type IV sensory neurons with blue light and then recording in motor neurons can help in explanation. A representative intracellular recording in muscles with sensory neurons stimulation is shown and can be used for instructive purposes. This line is UAS-ChR2-XXL;ppk-Gal4, and supplemented with ATR (1mM) produces robust responses in the muscle fibers (Figure 17).

Activation of ChR2 in type IV sensory neurons makes motor neurons to fire action potentials which in turn depolarize muscle fibers. The motor output (EPSPs traces) is being recorded while the third instar larva is being exposed to various intensity of blue light (see shading of blue light as intensity).
There are many additional types of behavioral assays that students may develop and try out. Another fun larval behavior is one in which the larvae are lined up on an agar dish and exposed to blue or white light and then the dish is placed in a dark spot or left exposed to dim room light. The larvae with ChR expressed in sensory or motor neurons demonstrate a paralyzed stance, which takes time to recover, as we illustrated in the module trial (Figure 18). The recovery time can be viewed by the movement away from the original line over time. Snap shots with a cell phone camera is an easy way for students to document movements over time.

When the larvae were exposed to regular light, they were all contracted and did not move (n=30, 10 larvae per agar plate per condition). 10 third instar larvae were placed on an apple juice agar plate. The larvae were exposed to regular light for 2 hours. The larvae stayed in their location without any movement. Three different conditions were used to show how much time it would take for the larvae to start moving again after 2 hour regular light exposure. The data shows that it takes about 15min for the larvae to restore their locomotor activity.
The use of the Arduino hardware and sample codes for flashing the LED light on and off is provided in Supplementary File 1. If instructors or students were so inclined to design experiments using the automated light controls for longer term studies on the effects of pulsing the lights on and off, this system is ideal, due to the low cost and ease in programing various codes. We are now using the system to teach subsets of freshman biology majors concepts of integrating engineering design with biological application at the University of Kentucky in the Department of Biology.
The exercises presented here promote investigations of practical neurobiological phenomenon in relation to human disorders (Parkinson’s, Stiff man syndrome, epilepsy, and autism), as well as promote discussion of potential medical interventions by pharmacological agents on these various neurotransmitter systems. An instructor might even have participants conduct a literature research and make predictions of the behavioral outcomes when stimulating the particular subsets of neurotransmitter systems for the larva and adult Drosophila before conducting the experiments on the flies. Establishing a conceptual model of the neurotransmitter and the neural circuits related to the mammalian behavior, and then testing if the model holds for Drosophila, is an important concept of the NGSS in the use of models and redesigning to observations (Krajcik & Merritt, 2012; NGSS Lead States, 2013). Titlow et al. (2015) and Pulver et al. (2011) used optogenetics and neurophysiological recordings with Drosophila for a college level educational activity with a similar context, which focused on neural circuits and synaptic function. Furthermore, body wall movements and adult behaviors can be recorded with a webcam (for example, WEBCAM HD4110, Hewlett-Packard Company, Palo Alto, CA, USA) connected to a computer, with a rate of 25 frames per second, for analysis outside of class time. See Titlow et al. (2014) for details in recording and analysis from captured data files.
Module instructors might wish to conduct pre- and post-assessment surveys, for students to provide their views of the exercises. The results of this brief survey is helpful for instructors to know what the students would likely know before the module starts and what might be gained from these exercises, since the module is intended to be an educational activity with in-depth content. A pre-assessment given a day before the laboratory experiments and the post-assessment given a few days after the exercises would be informative for instructors.
The pre- and post- assessment questions in Supplementary File 2 could be useful to future instructors.
In both high school and college settings, a very similar power point introduction of the lab exercises can be shown to students. This introduction should be given after the pre-assessment survey, so the presentation is part of the educational module. Instructors can decide on their own if they wish to use the power point content provided or modify for the level of the participants. The power point presentation we used for the trial module in a high school and a college class are provided as Supplementary File 3.
A high school teacher, who has >10 years of experience teaching high school Introductory Biology, Anatomy and Physiology, and Advanced Biology and has level one Biology certification, taught this module to sophomores to about 30 students high school students in Louisville (KY, USA). In addition, the teacher had received a MS in entomology prior to beginning a teaching career. These classes were 50 minutes in length and the class was divided into groups to work with different subsets of the exercises. After the data collection was completed, the various groups shared out the results with other class mates. Groups were divided into a pair of students and each group was given a different line of flies. Some were provided with the sensory lines while others with the motor drivers or serotonin lines. Each group conducted a larval body wall movement assay and an adult climbing assay. A second cohort of 15 senior high school students in Sommerset (KY, USA) was introduced to this educational module. The high school instructors were pleased to expose students to molecular biology in how the fly lines were produced with genetic manipulation, addressing neural circuits and allowing the students to produce various behavioral assays while collected data which later was graphed and discussed as a class. The teachers integrated this content along with teaching the nervous system, which was part of the normal curriculum.
The high school teacher’s comments were:
“Sometimes it is hard to focus the light on the larvae crawling on the dish.”
“Having the room lights off or very dim while the students work might be difficult to achieve in some classrooms.”
“Students might not understand the physiological concepts of how nerves and muscles work until they [have] covered this concept in a biology class”.”
These modules were taught in the beginning of the school year in a college level biology major class with juniors and seniors with about 120 students. The college teacher’s comments were as follows:
“Implementing this module for a senior college level biology class, which has a laboratory component, provided a different perspective than for high school students. The teaching instructors had a three hour period in one setting to explain and conduct the experiments. An aggregate of instructors’ comments were:
“A three hour lab is just about right for this series of experiments if various groups work on different fly lines.”
In summary, the presented exercises have been beta tested with students at different educational levels, and these students appear to be learning novel content and have an interest in learning. However, we have not quantified student learning assessments beyond the causal discussion with students. The instructors have provided informative feedback after implementing the activities, so modifications can be made for future classes such as addressing if the room can be dark enough to conduct the assays and if dissecting scopes are available to students to examine the 1st and 2nd instar larvae. The topics presented are rich in physiological history and show how the current state of biotechnology, engineering and science have merged into the ability of controlling the development of defined neural circuits that regulate animal behavior. The future applications for human disease states are just now being probed with this technology of optogenetics, so these exercises should be exciting to students and teachers, if they are made aware in the beauty in the integration of computer coding, biotechnology, and implications for neurobiology by embracing the content presented.
Dataset 1: Raw data for all module trial results. Sigma Plot version 13 files for Figure 4, Figure 6–Figure 8, Figure 10–Figure 12, Figure 14–Figure 16. doi, 10.5256/f1000research.10632.d150960 (Majeed, 2017).
ZM, FK, RLC conceived the study. ZM, FK, JM, HA, JW, RLC designed the experiments. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
ZRM supported by Higher Committee for Education Development (HCED) scholarship in Iraq. FK supported by Deutscher Akademischer Austausch Dienst (DAAD); German Academic Exchange Service; RISE - Program (Research Internships in Science and Engineering). Part funded by Kentucky Science and Engineering Foundation (KSEF-3712-RDE-019) at the Kentucky Science and Technology Corporation (RLC) and personal funds supplied by RLC.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary File 1: Use of the Arduino hardware and sample codes for flashing LED lights.
Click here to access the data.
Supplementary File 2: Pre and post-test educational sample assessment is provided.
Click here to access the data.
Supplementary File 3: Power point presentation used to introduce the exercises for the participating classes. The content can readily be modified by the instructor according to the level of instruction required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 08 Feb 17 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)