Keywords
home based, exercise, muscle mass, muscle function
home based, exercise, muscle mass, muscle function
Older age is associated with the loss of skeletal muscle mass. Termed sarcopenia, age-related muscle wasting is associated with loss of muscle strength (dynapenia), increased morbidity, loss of independence and premature mortality (Rudrappa et al., 2016). There are however proven means by which to offset these detrimental progressive declines. For example, structured and fully-supervised progressive resistance exercise training (RET) has been shown to improve both muscle mass (Phillips et al., 2012) and function (Liu & Latham, 2009).
Despite the established effects of RET on muscle mass and function, compliance is difficult to achieve, due to perceived (and real) socio-economic, psychological and environmental factors (Zlot et al., 2006). Almost all current evidence for the efficacy of RET is based on 2–3 sessions each week, for up to 12 weeks, at a gym or with specialist equipment, reflecting current physical activity guidelines for adults of 2–3 days per week RET for each of the major muscle groups (Bull et al., 2010). However, compliance to physical activity recommendations is poor, with recent data suggesting that they are only achieved by 38% of UK adults (Department of Health, Physical Activity, 2011), a figure that is lesser still in older adults (Jefferis et al., 2014). As a result, home-based exercise training (HBET) programmes have been suggested as an alternative to overcome some of the barriers associated with poor compliance to exercise (Hong et al., 2017; Silveira et al., 2013), whilst also providing a platform to develop the benefits of RET for (pre-) sarcopenic individuals (Maruya et al., 2016).
The majority of HBET studies to date, have focussed on pre- or rehabilitation for specific clinical groups (Bassett & Prapavessis, 2007; Chang et al., 2017). A small number of studies have specifically investigated the efficacy of HBET on skeletal muscle function in the elderly, although the focus of these studies has mainly been on lower limb exercises to improve walking and balance (Ito et al., 2015; Yamauchi et al., 2009) in relation to fall and fracture prevention. Other studies in older adults have incorporated the use of specialist aids e.g. dumbbells (Plotnikoff et al., 2010), elastic bands (Jette et al., 1999; Ribeiro et al., 2009) or ankle weights (Gardner et al., 2001) to provide resistance for HBET; rather than attempting to incorporate exercise into tasks of daily living, as is done herein. To date, no studies have attempted to investigate the efficacy of incorporating whole-body exercise into activities of daily living on muscle mass and function in older adults. Consequently, in this pilot study we aimed to assess the effects of a 4-week, progressive, whole-body HBET fully integrated into activities of daily living, on muscle mass and function in healthy older individuals. We also aimed to utilise a recently re-introduced (Clark et al., 2014) and underexploited creatine method to estimate whole-body muscle mass in humans.
Twelve healthy older male and female volunteers (63±3 y, 7 men: 5 women, BMI: 29±1 kg/m2) with no previous chronic disease history were recruited via an advertisement on a local radio station. Before enrolment into the study, all volunteers had an assessment of previous medical history and an ECG to check for any subclinical arrhythmias. The ECG’s were approved by a clinically qualified physician. All volunteers provided written informed consent prior to the start of this study. This study was approved by The University of Nottingham Medical School Ethics Committee (B28102015 SoMS GEM BBC) and conformed to The Declaration of Helsinki.
The study involved two visits by the study participants, before and after the 4-week HBET (Figure 1), to the University of Nottingham Medical School, at the Royal Derby Hospital. All assessments at these visits were conducted by researchers from the MRC-ARUK Centre for Musculoskeletal Ageing Research. At each visit, volunteers provided a baseline urine sample for the muscle mass assessment by D3-Creatine before undergoing measures of height, weight (Marsden Weighing Group, UK), resting heart rate and blood pressure (Omron M5-1 digital BP monitor, Omron, UK). Each volunteer had the thickness, fascicle length (Lf) and pennation angle (θpen) of their vastus lateralis muscle, and the cross-sectional area (CSA) of their quadriceps measured by ultrasound (Mylab 70, Esaote Biomedica, Genova, Italy), using the protocol described by Franchi et al. (2014). Volunteers then completed a number of muscle function assessments:
• maximal voluntary contraction (MVC) for seated leg extension and flexion using an isokinetic dynamometer (Isocom; Isokinetic Technologies, Eurokinetics, UK),
• leg extension 1-repetition maximum (1-RM; ISO leg extension, Leisure Lines Ltd., GB),
• leg power (Nottingham Power Rig; Bassey & Short, 1990),
• handgrip strength (Grip D 5401; Takei Scientific Instruments Co. Ltd., Japan); and
• a short physical performance battery test (SPPBT; Guralnik et al., 1994).
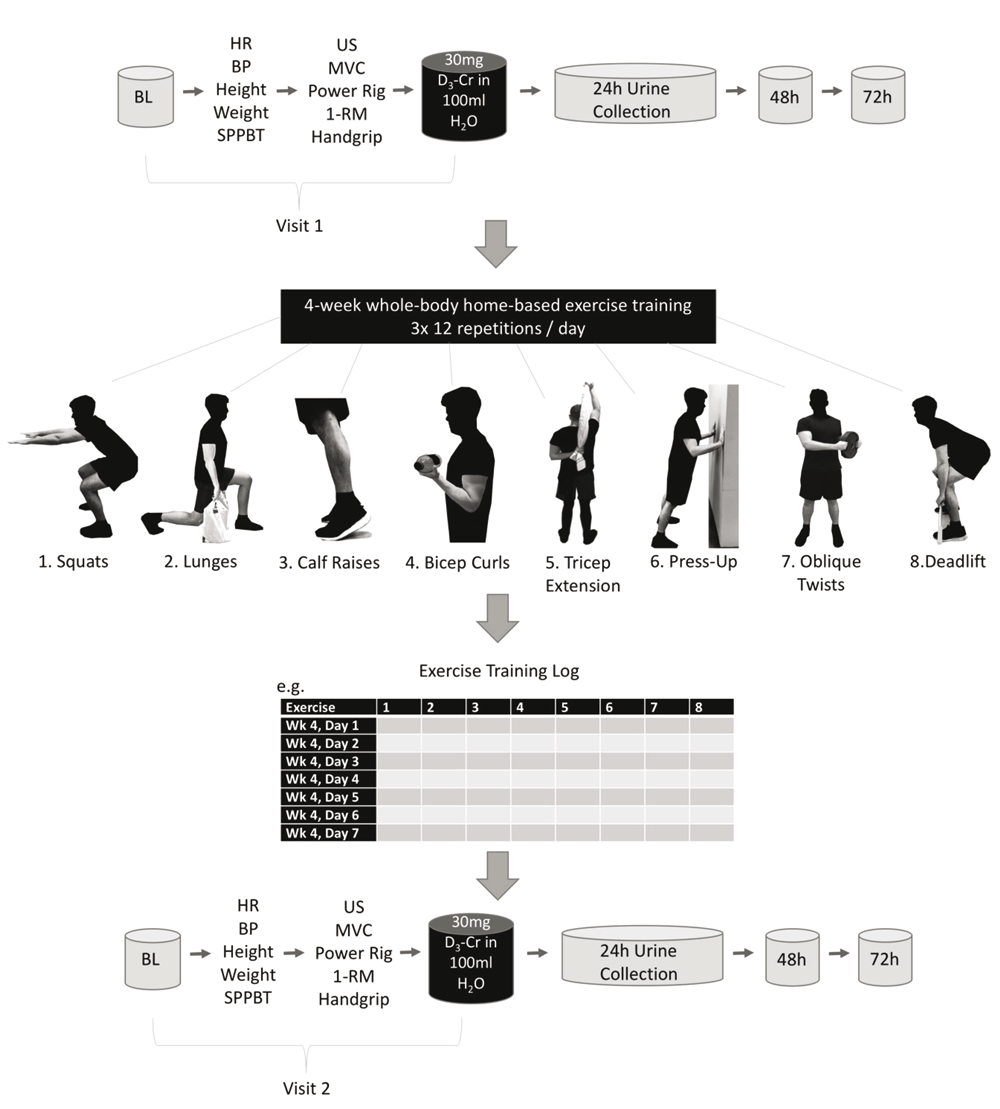
BL: baseline urine sample; HR: resting heart rate; BP: resting blood pressure; SPPBT: short physical performance battery test; US: leg ultrasound; MVC: maximum voluntary contraction; 1-RM: leg extension 1-repetition maximum; D3-Cr: D3-Creatine for whole-body muscle mass assessment; 48 and 72h represent the time of urine samples post-tracer consumption.
MVC was measured at two knee angles (60 and 90°), whilst isokinetic (eccentric/concentric) contractions were measured over a 70° range (20–90°) at three different speeds (60, 180 and 240°/sec). For both measures, horizontal seated leg extension was set at 0°. Following the muscle function assessments, volunteers ingested 30mg of D3-Creatine dissolved in 100ml H2O for muscle mass assessment, before providing a pooled 24h urine collection and single urine samples at 48 and 72 h post consumption (Clark et al., 2014).
Volunteers were required to complete 3 sets of 12 repetitions across 8 different exercises, every day, for 4 weeks. Each exercise was designed to be easily incorporated into activities of daily living to minimise the time-commitment required (e.g. bicep curls whilst cooking, see Figure 1). In total the exercise programme consisted of: squats, lunges, calf raises, bicep curls, triceps extensions, semi-incline press-ups, oblique twists and deadlifts. Participants were instructed to take 2 seconds to perform each phase (eccentric and concentric) of each exercise and to hold for 2 seconds at the mid-point of each exercise. Progression via increased resistance or time-under-tension was encouraged once the full complement of repetitions could be completed. Compliance to exercise was measured by self-report (see self-report log in Figure 1). An exercise would only be marked as complete if 3 sets of 12 repetitions had been performed within a day.
Using a creatine-tracer measure of muscle mass (Clark et al., 2014), D3-creatine and D3-creatinine enrichment, plus unlabelled creatine and creatinine in the urine was measured using high-performance liquid chromatography/mass spectrometry (HPLC/MS), as detailed in (Stimpson et al., 2012). Total muscle mass was calculated using the following equation:
Total muscle mass
where MWUnlabelled and MWlabelled represents the molecular weights of both unlabelled and labelled creatine, respectively. The estimated creatine pool size is then divided by 4.3 g/kg, which reflects the concentration of creatine in wet muscle.
Descriptive statistics were carried out on all datasets and checked for a normal distribution using the Shapiro-Wilk normality test. Datasets were analysed using paired Student’s t test to assess the effect of HBET, with GraphPad Prism Software v5 (La Jolla, CA, USA). All data is presented as mean ±SEM. Significance was set at P<0.05.
Using data from the self-report training logs, overall compliance to the HBET was 87.3±3.0% over the 4-week period of training. Adherence to each individual exercise was >86%, with the calf-raises being the most completed exercise (91%). No significant changes were found following HBET for body weight (79.3±4.7 vs. 78.9±4.6 kg), resting heart rate (71.9±3.1 vs. 71.4±3.9 bpm) or blood pressure (systolic: 144.4±3.4 vs. 140.7±4.2, diastolic: 92.2±2.5 vs. 89.0±2.4; average of 3 measurements).
HBET did not elicit significant changes in leg extension 1-RM, handgrip strength or SPPBT; there was however, a trend for an increase in leg extension 1-RM (45.7±5.9 vs. 49.6±6.0 kg, P=0.08; Figure 2A). HBET did elicit significant increases in leg power (276.7±38.5 vs. 323.4±43.4 W, P<0.05; Figure 2B) and MVC at 60° (154.2±18.4 vs. 168.8±15.2 Nm, P<0.05; Figure 2C) and 90° (152.1±10.5 vs. 159.1±11.4 Nm, P<0.05; Figure 2D). There were also significant increases in peak torque recorded at 60°/sec during both seated flexion (74.7±8.3 vs. 80.8±8.6 Nm, P<0.05; Figure 2E) and extension (114.9±10.2 vs. 123.8±9.4 Nm, P<0.01; Figure 2F) after HBET.
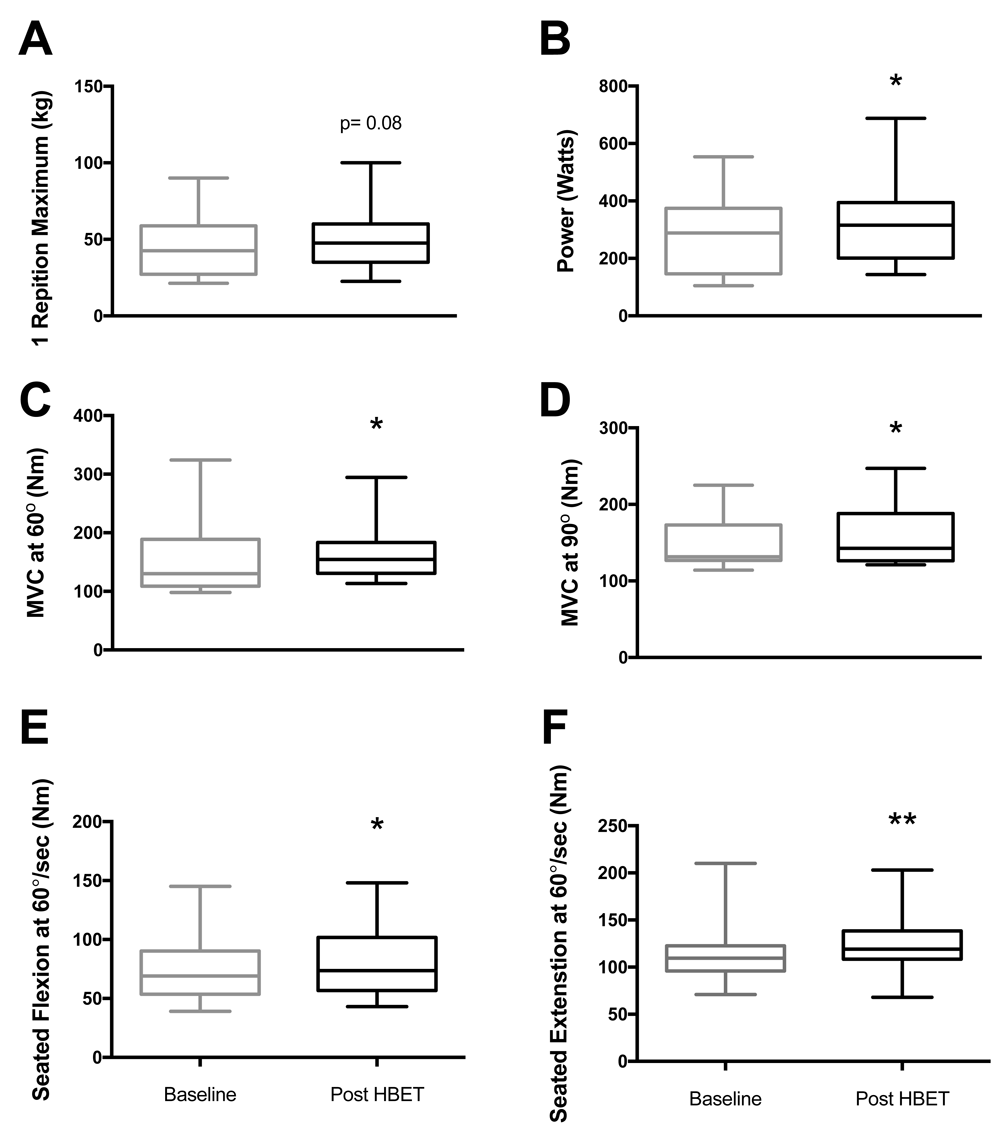
A) 1-RM leg extension (45.7±5.9 vs. 49.6±6.0 kg, P=0.08), B) Leg power via Nottingham Power Rig (276.7±38.5 vs. 323.4±43.4 W), C) MVC at 60° (154.2±18.4 vs. 168.8±15.2 Nm), D) MVC at 90° (152.1±10.5 vs. 159.1±11.4 Nm), E) Seated leg flexion at 60°/sec (74.7±8.3 vs. 80.8±8.6 Nm), F) Seated leg extension at 60°/sec (114.9±10.2 vs. 123.8±9.4 Nm). Baseline vs. post-HBET (mean ± SEM), n=12, *=p<0.05; **=p<0.01. MVC: Maximal voluntary contraction.
CSA of the thigh at 50% of thigh length significantly increased (57.5±5.4 vs. 59.0±5.3 cm2, P<0.05; Figure 3A) after HBET. However, there was no significant increase in muscle thickness, fascicle length (Lf) or pennation angle (θpen) of the m. vastus lateralis. There was a trend for an increase in muscle thickness (25.0±1.6 vs. 25.5±1.7 mm, P=0.08; Figure 3B) after HBET.
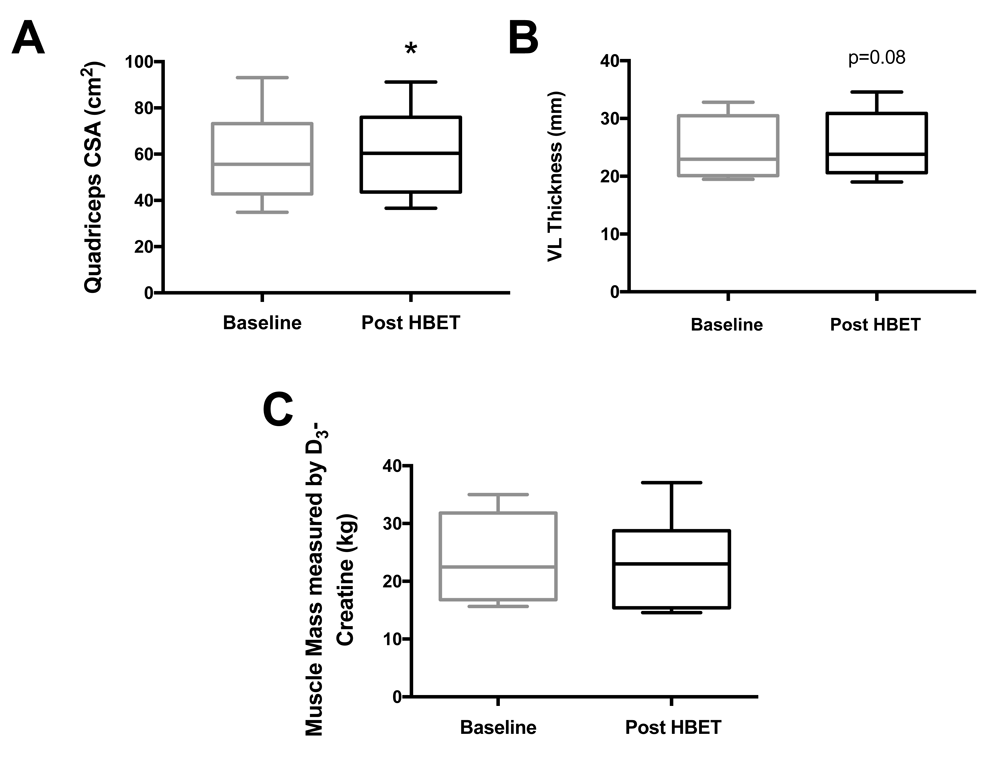
A) quadriceps cross-sectional area (CSA; 57.5±5.4 vs. 59.0 ± 5.3 cm2); B) m. vastus lateralis (VL) thickness (25.0±1.6 vs. 25.5±1.7 mm, P=0.08); C) whole-body muscle mass, before and after home-based exercise training (HBET) (23.9±2.1 vs. 22.8±2.2 kg). (mean ± SEM), *=p<0.05, baseline vs. post-HBET.
Corresponding with no significant change in body weight, there was no significant difference in whole-body muscle mass as estimated by D3-Creatine (Figure 3C) after HBET (23.9±2.1 vs. 22.8±2.2 kg).
In this pilot study, we show that lifestyle-integrated, whole-body HBET has the potential to meaningfully improve muscle function in older adults. We have shown that this training mode can increase leg power by 16.9% in just 4-weeks. Although lower than the 37% power increase demonstrated in a solely lower-extremity focused HBET programme, this study was conducted over an 8-week period (DeBolt & McCubbin, 2004), suggesting that if the magnitude of increase was to continue at a constant rate, our training mode may elicit similar improvements. Additionally, we demonstrate increases in muscle strength (via MVC at 60°) similar to that observed in response to a 6-week, fully-supervised, gym-based training study (Brook et al., 2016). Furthermore, despite no significant increase, there was a positive trend in the leg extension 1-RM, similar to that seen in an 8-week HBET study by Zion et al. (2003). Thus, while muscle function adaptations to HBET were numerically lesser compared to longer-term structured exercise regimens (some requiring specialist equipment), our data support the notion that just 4-weeks of a ‘lifestyle-integrated’ HBET meaningfully improves muscle function.
Despite these positive results, no changes in handgrip strength were found, a similar result to Nelson et al. (2004), who implemented a 6-month home-based whole-body exercise programme in 70 male and female elderly individuals. This may be due to a lack of exercises specifically targeting the forearm flexors. Indeed, although it is well-established that extant grip strength represents a biomarker of muscle function in older adults (Bassey & Harries, 1993), our data suggests that it is an insensitive biomarker for important functional and mass gains in other muscle groups (e.g. the legs) following certain exercise interventions.
Importantly, increases in thigh CSA (and a trend towards increased muscle thickness), which may partly explain the functional improvements, were observed. Measures of thigh CSA by ultrasound have been positively correlated with those by MRI (Reeves et al., 2004), therefore suggesting (leg) muscle hypertrophy in this study. However, since this occurred in the absence of detectable changes in whole-body muscle mass as measured by D3-Creatine, we speculate i) that preferential hypertrophy of leg muscles occurred, but remained undetectable on a whole-body level; or ii) that measurement of whole-body muscle mass was below the detection threshold for the D3-creatine method.
We acknowledge limitations to our study design. The use of validated physical activity monitors (e.g. Actiheart, CamNtech, Cambridge, UK) to assess compliance to exercise would have provided greater information on the adoption of, and adherence to our exercise programme. Similarly, the use of interactive technologies (e.g. training applications for tablets/smartphones) for motivation and/or instruction may have improved adherence (Silveira et al., 2013). The lack of improvements in SPPBT were likely a result of our volunteers achieving optimal scores before the intervention.
To conclude, our findings demonstrate that significant increases in muscle mass, maximal voluntary contraction, maximal power and isokinetic strength can be achieved with just 4-weeks of unsupervised, lifestyle-integrated HBET in older adults. These improvements were achieved via cost-effective means, without the requirement for specialist equipment, facilities or supervision (DeBolt & McCubbin, 2004; Jette et al., 1996; Zion et al., 2003). With high compliance and beneficial physiological effects in only a 4-week period, this study highlights the potential for lifestyle-integrated HBET to improve skeletal muscle ‘health’ in older adults. As increases in muscle mass and strength are positively correlated with overall physical function (Liu & Latham, 2009), this novel training mode may be of particular benefit to older frail/(pre-) sarcopenic adults.
Dataset 1: Raw data supporting the findings in this study.
Sheet 1: Maximum Voluntary Contraction (MVC) at 60° and 90°, pre- and post 4-weeks of home-based whole-body exercise training.
Sheet 2: Seated leg extension measures at 60, 180 and 240 deg/sec pre- and post 4-weeks of home-based whole-body exercise training
Sheet 3: Seated leg flexion at 60, 180 and 240 deg/sec pre- and post- 4-weeks of home-based whole-body exercise training
Sheet 4: Handgrip strength, single-leg 1-RM leg extension, SPPBT (short physical performance battery tests) and leg muscle power, measured pre- and post- 4-weeks of home-based whole-body exercise training
Sheet 5: Participant characteristics. Heart rate (HR), systolic and diastolic blood pressure (BP) and body weight, pre- and post- 4-weeks of whole-body home-based exercise training.
Sheet 6: Quadriceps cross-sectional area (CSA), vastus lateralis muscle thickness, fascicle length and pennation angle measured by ultrasound pre- and post- 4-weeks of whole-body home-based exercise training.
Sheet 7: Muscle mass measured by using D3-creatine, pre- and post- 4 weeks of whole-body home-based exercise training.
DOI, 10.5256/f1000research.11894.d170288 (Cegielski et al., 2017).
We would like to thank MRC-ARUK for providing centre funding (MRC: MR/K00414X/1 and ARUK: 19891) and supporting this work. The stable-isotope analysis in this work was funded by Medical Research Council Confidence in Concept Awards (CiC12019 and CiC2014035). The Abbeyfield Research Foundation, charity number 1167685 (RGS117347) supports PJA, BEP and KS at the University of Nottingham.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Tieland M, Verdijk LB, de Groot LC, van Loon LJ: Handgrip strength does not represent an appropriate measure to evaluate changes in muscle strength during an exercise intervention program in frail older people.Int J Sport Nutr Exerc Metab. 2015; 25 (1): 27-36 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Exercise, muscle, training, ageing.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 11 Sep 17 |
read | |
|
Version 1 26 Jul 17 |
read | |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)