Keywords
breast cancer, breast cancer marker, P63, SOX2, triple negative breast cancers
breast cancer, breast cancer marker, P63, SOX2, triple negative breast cancers
We revised the introduction focusing on TNBC epidemiology. We emphasised the introduction related to TNBC classification, characteristics and behaviour. We also updated the role of p63 in cancer within the introduction.
In the conclusion, we included more information related to the frequency of Basal and Non-basal type of TNBC and this finding related to the expression of SOX2 and P63 both in Basal and Non-basal type of TNBC.
We also included the acknowledgement section in the current version. We also revised the author list; Harapan Harapan, MD was requested to be mentioned in the acknowledgement section rather than as co-author due to current changing of his research field of interest.
See the authors' detailed response to the review by Irianiwati Widodo
See the authors' detailed response to the review by Diah Rini Handjari
Breast cancer, accounting for 25% of all cancer cases and 15% of all cancer deaths among females, is the most frequently diagnosed cancer among female worldwide1,2. The incidence of breast cancer increased significantly, approximately by 30% in developed countries3 and currently it has been also rising in many developing countries2. In Asia, 639,824 breast cancer cases and 228,926 deaths were recorded in 2012, from which 48,998 cases and 19,750 deaths occurred in Indonesia4. Triple-negative breast cancer (TNBC), a group of breast cancers with the absence of oestrogen receptor and progesterone receptor and no overexpression of human epidermal growth factor receptor 2 (HER2), represents 10%–20% of invasive breast cancer. A global data base, National Cancer Data Base (NCDB), reveals that TNBC was present in 13% of breast cancer patients, ranged from 23.7% in African-Americans to 8.9% in Filipino patients5. In Southeast Asia, a study found that TNBC presented in 10.5% among 1227 breast cancer patients6 . In Indonesia, a study that was conducted between 2010 and 2011 in Bandung found that 11.9% of breast cancer patients were TNBC7.
Immunohistochemically, TNBC could be divided into two subtypes: Basal-like (positive for the expression of high-molecular-weight/basal cytokeratins 5/6 (CK5/6) and epidermal growth factor receptor (EGFR)) and Non basal-like (negative for the expression of CK5/6 and EGFR). Basal-like TNBC usually has p53 mutation, EGFR overexpression, loss of function of BRCA1, c-MYC amplification, and high histological grade indicating more aggressive characteristics and aggressive behavior8,9. In addition, majority of Basal-like cancer cannot be managed effectively with trastuzumab and hormonal treatments10.
Advanced screening and diagnosis methods for breast cancer such as mammograms, ultrasound, magnetic resonance imaging and fine-needle aspiration, have allowed for detection of small lesions at the early stage. Identifying breast cancer at the early stage will increase the potential for curative treatment and therefore increases the survival rate11–14. However, smaller lesions are more challenging to diagnose. Therefore, it is essential to use an advanced immunohistochemical approach for evaluation of smaller tissue specimens that target more specific markers15.
A previous study using a MCF7 breast cancer cell line to produce MCF7-derived tumour xenografts found that P63 and SOX2 immunostainings were two potential markers for breast cancer16. P63, involved in cellular differentiation, is a homolog of tumour protein P53 and in normal breast ducts and lobules it is expressed frequently in the nuclei of myoepithelial cells17. Mutation of the p53 gene results in a very high risk of breast cancer18. The roles of p63 in tumorigenesis, cancer progression, and metastasis are still being discovered. However, in animal model found that p63 deficiency may be a causative factor for metastatic spread19,20. In addition, clinical evidence suggests that a robust correlation between reduced p63 expression and cancer progression21.
A study revealed that the total percentage of P63-positive cells was related to marked nuclear pleomorphism and the intensity of P63 staining was associated with syncytial growth pattern in TNBC22. In addition, data also reveals that p63 gene expression in breast cancer could be used as a specific marker of metaplastic carcinoma17, and P63 immunohistochemical staining could improve diagnostic accuracy of breast cancer even in small tissue specimens23.
SOX2 is a transcription factor belonging to the SOX family and functions as an activator or suppressor of gene transcription24,25. Data shows that SOX2 promotes cellular proliferation of breast tissue26 and regulates self-renewal in cancer stem cells27. The scientific evidence reveals that SOX2 acts as an oncogene in epithelial cancers25 and in the breast, a study found that silencing of sox2 gene was associated with reduction of the size of the cancer stem cells and restoration of tamoxifen sensitivity28. All together, these data indicate that P63 and SOX2 have pivotal role in breast cancer and therefore are potential to be used as specific biomarkers. This study was conducted to assess the immunoexpression of P63 and SOX2 in TNBC cases in order to provide insight regarding their potential diagnostic value (single or in combination) to differentiate TNBC types.
A cross-sectional study to assess the immunoexpression of P63 and SOX2 in TNBC cases (negative expression of estrogen and progesterone receptors and c-erbB2) was conducted. Histological slides of TNBC and their paraffin blocks, tested between the 1st of January 2011 and 31st of December 2015, were collected from the Pathology Anatomy Laboratory, Dr. Hasan Sadikin Hospital, Bandung, Indonesia. Each histological slide was examined by two certified pathologists. To classify the type of TNBC morphology, between Basal-like type TNBC and Non basal-like type TNBC, cytokeratin 5/6 (CK 5/6) immunohistochemical staining was carried out on all TNBC histological slides. Concurrently, the immunoexpression of P63 and SOX2 was measured using immunohistochemical stains with specific primer antibodies. The protocol of this study was approved by the Health Research Ethical committee of Sumatera Utara University (approval 103/KOMET/FK USU/2015) and the usage of histological specimens was approved by the Pathology Anatomy Laboratory of Dr. Hasan Sadikin Hospital (LB.02.01/B29/239/X/2015).
Forty archival paraffin blocks from TNBC cases were subjected to immunohistochemical staining to assess the immunoexpression of CK 5/6, P63 and SOX2. Briefly, 4 μm sections of each paraffin block were prepared using standard procedure29. Immunohistochemical staining was conducted using primary antibodies as follows: anti-CK5/6 monoclonal antibody (Biocare Medical, Concord, CA, USA), anti-P63 monoclonal antibody (Biocare Medical, Concord, CA, USA) and anti-SOX2 monoclonal antibody (Abcam, Cambridge, UK). Starr Trek Universal HRP Detection (Biocare Medical, Concord, CA, USA) was used as second antibody. A chromogen 3,3’-diaminobenzidine (DAB) (Biocare Medical, Concord, CA, USA) was used to develop the colour. For each experiment, appropriate controls were used.
Immunoexpression of CK 5/6 was interpreted as positive or negative, in which positive CK 5/6 indicates Basal-like type TNBC while negative CK 5/6 indicates Non basal-like type TNBC. Immunoexpression of P63 and SOX2 was evaluated using an immunoreactivity scoring system that had been published elsewhere with modification22. Staining intensity was scored as follows: 1 (no staining), 2 (weak staining), 3 (moderate staining) and 4 (strong staining). The percentage of positively stained tumour cells was assessed as a proportion of the total number of tumour cells present in the section as follows: 1 (<20%), 2 (≥20–50%), 3 (>50–80%) and 4 (>80%).
Immunoreactivity score was calculated by multiplying staining intensity and the percentage of positivity, and the score therefore ranged from 1 to 16. The immunoreactive score was then divided into low (≤ 5), moderate (≥ 6 – 10) and high (≥11 – 16). Immunoexpression of P63 was measured both in cytoplasm and nucleus while SOX2 immunoexpression was measured in nucleus only.
Normality of the data was assessed using the Shapiro-Wilk test and therefore the analysis tests chosen based on the normality of the data. The correlations between immunoexpression of P63 (cytoplasm and nucleus) and SOX2 were assessed using Pearson correlation and Spearman correlation, respectively. The associations of P63 and SOX2 immunoexpression and type of TNBC were assessed using Mann Whitney test. The predictive diagnostic values of P63 cytoplasm for diagnosing Basal-like type TNBC were estimated using several immunoreactivity score cut-off points. Receiver operating characteristic curve (ROC) was plotted and area under the ROC curves (AUC) was estimated. For all analyses, estimates were considered statistically significant for two-tailed values of p<0.05. All analyses were conducted using Statistical Package for the Social Sciences software (SPSS for Windows, Version 16, Chicago, IL).
The histopathology of the TNBC samples used in this study is described in Table 1. Approximately 45% of the samples were classified as metaplastic carcinomas. In addition, immunohistochemical staining for CK 5/6 revealed that 23 (57.5%) of samples were Basal-like type TNBC and while 17 (42.5%) samples were Non basal-like type TNBC.
Immunoexpression of P63 and SOX2 in samples, categorized by immunoreactivity score, are presented in Table 2. For both types of TNBC (basal and non basal-like type), all immunoreactivity scores for P63 in the nucleus were classified as low grade, while 11 (27.5%) and 7 (17.5%) samples were classified as moderate and high grade, respectively for the P63 in the cytoplasm. The immunoreactivity grade for SOX2 was similar to P63 in the cytoplasm, and therefore correlation analyses were conducted.
There was a strong negative correlation between immunoexpression of P63 in the cytoplasm and immunoexpression of SOX2 in the nucleus in metaplastic carcinoma (a sub-type of TNBC basal-like type) (r=-0.73, p=0.013) (Table 3). In addition, linear regression showed a relatively strong correlation between P63 cytoplasm and SOX2 immunoexpression in metaplastic carcinoma (r=0.49, p=0.012). There was no significant correlation between P63 cytoplasm and SOX2 immunoexpression in Non basal-like type TNBC, and no significant correlation between P63 nucleus and SOX2 immunoexpression either in Basal-like type or Non basal-like type of TNBC.
| TNBC basal-like type | n | P63 | SOX2 | r | p |
|---|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | ||||
| Invasive ductal carcinoma | 2 | 5.50 (3,54) | 5.00 (5.66) | - | - |
| Invasive ductal carcinoma and invasive lobular carcinoma | 1 | 16.00 (0.00) | 9.00 (0.00) | - | - |
| Invasive lobular carcinoma | 1 | 8.00 (0.00) | 1.00 (0.00) | - | - |
| Invasive micropapillary carcinoma | 1 | 12.00 (0.00) | 16.00 (0.00) | - | - |
| Medullary carcinoma | 6 | 5.50 (4.76) | 8.00 (5.48) | 0.64 | 0.172 |
| Metaplastic carcinoma | 12 | 6.67 (4.68) | 6.00 (3.77) | -0.73 | 0.013* |
Immunoexpression of P63 cytoplasm, P63 nucleus and SOX2 in Basal-like and Non Basal-like TNBC is shown in Table 4. The data indicates that the immunoexpression of P63 cytoplasm in Basal-like type TNBC was significantly higher compared to Non basal-like type TNBC (p=0.021). Immunoexpression of P63 nucleus and SOX2 was not different between Basal-like and Non basal-like types of TNBC, with p-values of p=0.27 and p=0.17, respectively.
| Marker | TNBC type | n | Immunoreactivity score Mean (±SD) | p |
|---|---|---|---|---|
| P63 cytoplasm | Basal-like | 23 | 6.96 (4,73) | 0.021* |
| Non basal-like | 17 | 3.76 (4,16) | ||
| P63 nucleus | Basal-like | 23 | 1.22 (0,52) | 0.273 |
| Non basal-like | 17 | 1.06 (0,24) | ||
| SOX2 | Basal-like | 23 | 6.78 (4,69) | 0.172 |
| Non basal-like | 17 | 4.82 (3,61) |
As mentioned above, immunoexpression of P63 in the cytoplasm was the only marker that was significantly different between TNBC types. Therefore, immunoreactivity score of P63 cytoplasm was further analysed to determine its ability to predict Basal-like type TNBC. Table 5 shows the predictive values of P63 in the cytoplasm for determining Basal-like type TNBC, using seven immunoreactivity score cut-off values from 3 to 9. It shows that P63 has relatively weak diagnostic value in diagnosing Basal-like type TNBC. The highest sensitivity was achieved at immunoreactivity score 3, while specificity was increasing with a higher immunoreactivity score.
Using the average score of P63 cytoplasm immunoexpression for Basal-like type TNBC in this study, 5.6 or 6, the sensitivity and specificity of P63 cytoplasm immunoreactivity score to predict Basal-like type TNBC was 56.5% and 72.6%, respectively with area under curve 0.64. The receiver operating curve of predictive diagnostic value of P63 cytoplasm for determining Basal-like type TNBC is plotted in Figure 1.
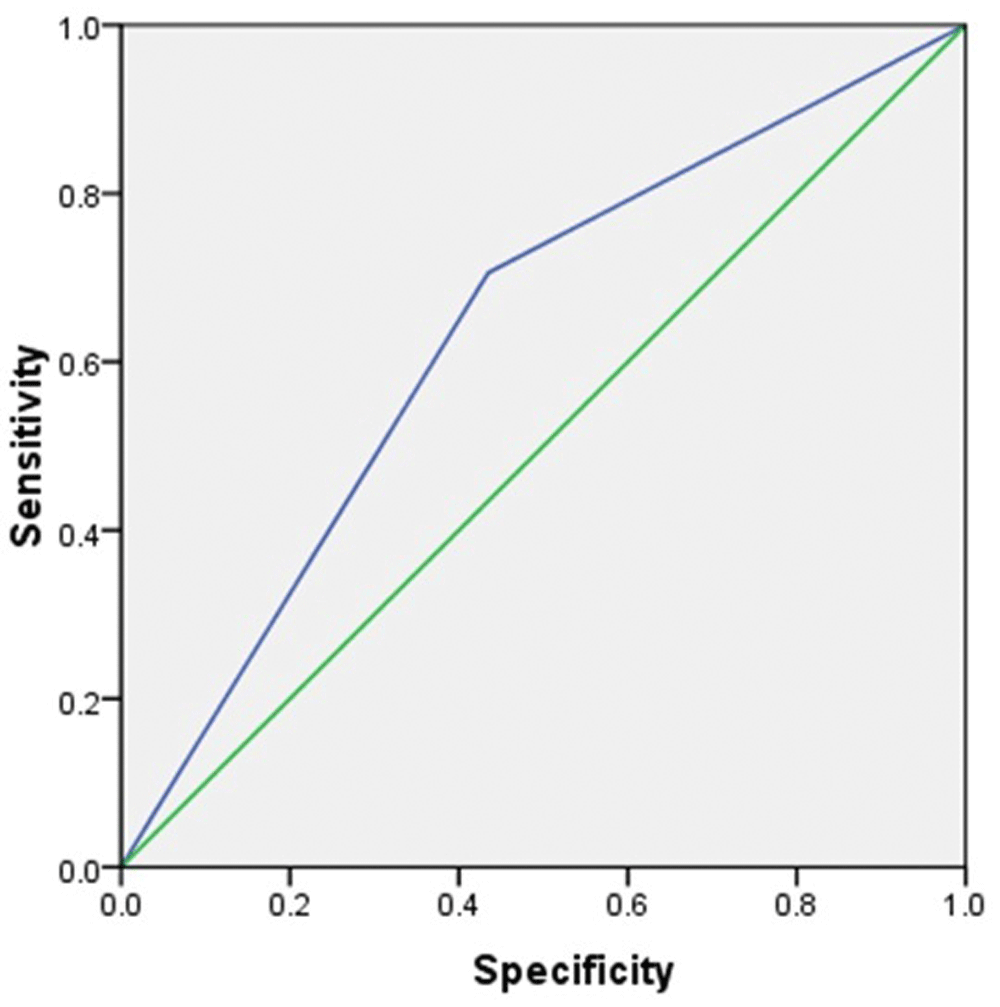
To the best of our knowledge, this is the first study conducted to assess the immunoexpression of P63 and SOX2 in TNBC cases in Indonesia. Some studies have been conducted to assess the predictive values of P63 as specific marker for breast cancer17,30. In addition, the idea of utilization of a cocktail of specific markers has been proposed previously to provide higher sensitivity and specificity for diagnosing specific breast cancers15,22,31. However, none of the previous studies had been conducted to assess the diagnostic value of immunoexpression of P63 and SOX2 in combination. This study, at the beginning, sought to assess predictive value of combination both of those markers for specific type of TNBC. However, we found that there was no difference in immunoexpression of SOX2 between Basal-like type TNBC and Non basal-like type TNBC. Nevertheless, we found that immunoexpression of P63 cytoplasm, but not P63 nucleus, was higher in Basal-like type TNBC compared to Non basal-like type TNBC.
P63 has been proposed as a breast cancer marker for a long time, but with conflicting results. A study demonstrated that immunoexpression of P63 was associated with breast cancers, for example the metaplastic carcinoma type of breast cancer17, but there was no difference in immunoexpression of P63 between medullary breast carcinomas and atypical medullary breast carcinomas of TNBC30. In our study, we found that the sensitivity and specificity of P63 cytoplasm immunoexpression to diagnose Basal-like type TNBC was 56.5% and 72.6%, respectively, with area under curve of 0.64. This sensitivity and specificity seems higher compared to a previous study, with 14% and 94%, respectively in determining a Basal-like type in infiltrative ductal carcinomas (TNBC)22. All together, these data indicate a weak predictive value of P63 immunoexpression as marker for Basal-like type TNBC. However, a study found that P63 is a specific marker for metaplastic carcinomas of the breast (a sub-type of Basal-like type TNBC)17. In our study, we could not assess the predictive value of P63 cytoplasm immunoexpression for determining metaplastic carcinomas, due to the small sample size (see Table 3).
We found that SOX2 immunoexpression grade was classified as moderate and high grade in 55% of TNBC cases (Table 2,) and it has been indicated previously that SOX2 has strong roles in promoting breast cancers26–28,32. However, there was no different in immunoexpression between Basal-like type TNBC and Non basal-like type TNBC. This result indicates that SOX2 expression is not different amongst TNBC types. This finding was in line with a previous study that indicated that SOX2 was expressed across different breast cancer subtypes33. A study found that SOX2 antibody in the sera was is higher in patients with breast cancer compared to healthy women and therefore it could be used to discriminate between breast cancer patients and healthy controls34. In addition, a meta-analysis found that SOX2 expression was associated with tumor size, histological grade, the aggressiveness and lymph node metastasis in TNBC patients35. All together, these results indicate that there was a possibility SOX2 expression could be used for diagnosing breast cancers, but there was no difference in expression amongst breast cancer types, and therefore it could not be used as specific marker for differentiating TNBC types.
There are some limitations to this study. The sample size was relatively small, and therefore some analyses could not be conducted. In addition, the diagnostic specimens were collected from different procedures such as from biopsy, mastectomy or lumpectomy, and this might influence the immunoexpression of the markers.
Immunoexpression of P63 cytoplasm is higher among Basal-like type TNBC compared to Non basal-like type TNBC. However, the predictive diagnostic value of P63 immunoexpression in the cytoplasm for Basal-like type TNBC is relatively low, with 56.5% sensitivity and 72.6% specificity.
Dataset 1: Immunoexpression and immunoreactivity scores of P63, SOX2 and CK 5/6 in the forty specimens that were analysed. DOI, 10.5256/f1000research.12671.d17913136
We would like to thank Harapan Harapan, MD (https://orcid.org/0000-0001-7630-8413) from Medical Research Unit, School of Medicine, Syiah Kuala University, who assisted in revising the manuscript and provided intellectual contribution to this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pathologist with major interest in colorectal cancer
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a pathologist with major interest in breast cancer
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 08 Jan 18 |
read | read |
|
Version 1 28 Sep 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)