Keywords
Trichoderma, Pseudomonas fluorescence, Bacillus subtilis, Ralstonia solanacearum, biocontrol agent, bio-efficacy, bacterial wilt, tomato
Trichoderma, Pseudomonas fluorescence, Bacillus subtilis, Ralstonia solanacearum, biocontrol agent, bio-efficacy, bacterial wilt, tomato
Dr. Sushil Thapa confirms that the author has an appropriate level of expertise to conduct this research, and confirms that the submission is of an acceptable scientific standard. Dr. Sushil Thapa declares he has no competing interests. Affiliation: Texas A&M AgriLife Research, Amarillo, TX 79106, USA.
Bacterial wilt caused by Ralstonia solanacearum (formerly called Pseudomonas solanacearum) is a major bacterial disease for tomatoes. It invades the roots of diverse plant hosts from the soil and aggressively colonizes the xylem vessels, causing bacterial wilt disease1–3. It is a devastating plant disease most commonly found in tropical, subtropical and warm-temperate regions4,5. R solanacearum produces several known virulence factors, including extracellular polysaccharide (EPS), and a combination of plant cell wall-degrading enzymes, such as endoglucanase (EG) and polygalacturonase (PG). Mutants lacking EPS and EG shows reduced virulence6–8. The major virulence factors for this pathogen are plant cell wall-degrading polygalacturonases (PGs)9. Various biocontrol agents are used to control the bacterial wilt caused by R. solanacearum. Trichoderma harzianum10,11, Trichoderma viride12, Trichoderma asperellum13, Trichoderma virens14, Pseudomonas fluorescence15,16 and Bacillus subtilis17 are used as biocontrol agents to control bacterial wilt. Combination treatment methods using two or more of these agents are more effective in managing the disease than treatment using a single biocontrol agent10,18,19. Chemical bactericides and fungicides induce resistance in pathogens during long term use, which ultimately makes the pathogen tolerant to these chemical applications20–23. Hence, there should be a focus on the use of biological methods to control plant disease.
This study focuses on evaluating the efficacy of different native isolates of Trichoderma species, B. subtilis and P. fluorescence against bacterial wilt disease caused by the pathogen R. solanacearum in the tomato plant. The study hypothesizes that the native isolates of Trichoderma spp., B. subtilis and P. fluorescence can be used as bioantagonistic agents to control bacterial wilt (R. solanacearum) of tomato. This study tries to reflect the bioantagonistic effects by the microscopic examinations, agar well diffusion technique and by applying the concentrate of biocontrol agents in tomato plots, to calculate their efficiency by comparing them with chemical methods of treatment.
The sample collection and field trials were done at Agro Narayani Farm, Sukranagar, Chitwan District, Nepal (Latitude: 27.582016 and Longitude: 84.272259) where the bacterial wilt infection was recorded in the previous harvest. The tests in the field were conducted from 11 March 2017 to 8 July 2017 for 120 days in new transplants. At the time of transplant, compost fertilizer at the rate 2.94 kg/m2, urea at the rate of 23.59 gm/m2, potash at the rate of 29.49 gm/m2, DAP 19.66 gm/m2, borax at the rate 1.97 gm/m2 and zinc at the rate 1.97 gm/m2 were applied. At 45 days and 90 days of transplant NPK 20:20:20 was applied at the rate 9.83/m2. Spacing of the plant was 50 cm in double row system of 50 × 50 cm. The plot size was 50 m2 and total of 8 plots were used having 100 plants per plot. Weather data were also collected from online resources as reference.
Physical symptoms, such as wilting of young leaves, discolored tissue at the dissected part of the stem base, and white, slimy ooze when the dissected part of the plant was kept in the glass of water, were used for the identification of infected plants24.
Bacterial wilt infection in tomato plants (Variety: Manisha) grown in Chitwan, Nepal, was positively identified by S. Yendyo of Kishan Call Center (KCC). Six whole plants were brought to the Quality Control laboratory of Agricare Nepal Pvt. Ltd. in a sterile bag for the isolation of the pathogen.
R. solanacearum was isolated from dissected sections of the infected tomato plants on Casamino acid-Peptone-Glucose (CPG) Agar (casein hydrolysate 1 g/l, peptone 10 g/l, glucose 5g/l, agar 15g/l). Briefly, the stems of the infected plant were washed three times with autoclaved distilled water and then blot dried. After drying the stem were washed with 80% ethanol solution, then 1% sodium hypochlorite (NaOCl) solution was applied for 2 minutes. Final washing was done with autoclaved distilled water three times. The xylem of 2–3 cm from the stem was dissected and the sap was rubbed in CPG medium and inoculated for 48h at 28°C. Identification was done on the basis of the morphology of the colony on CPG medium, Gram staining and microscopic examination25,26.
A total of 13 strains were used in this study (see Table 1). Six strains of Trichoderma spp., two strains of Pseudomonas fluorescence and one strain of Bacillus subtilis were isolated from soils and root soils collected from different sites in Nepal. ATCC 13525 strain of P. fluorescence from Microbiologics, St. Cloud, MN 56303, France was used as the reference for isolated Pseudomonas species. Trichoderma harzianum, Trichoderma virens and Trichoderma asperellum strains were provided by Tamil Nadu Agricultural University (TNAU), Coimbatore, Andhra Pradesh, India, and were used as reference species for isolated Trichoderma spp.
This includes the short code given to the isolates for identification in this study.
Trichoderma spp. were isolated by serial dilution method using TSM agar plate (K2HPO4: 0.9 g/l, MgSO4: 0.2 g/l, KCl: 0.15 g/l, NH4Cl: 1.05 g/l, Glucose 3 g/l, Rose Bengal: 0.15 g/l, agar 20 g/l, streptomycin: 100 mg/l, tetracycline: 50 mg/l)27. 1 gm of each soil sample were suspended in 9 ml of sterile distilled water and vortexed (Accumax India, New Delhi-110058, India) for 5 min. The soil suspension was then serially diluted to 10-3 and 10-4. Pour plate technique was used by mixing 1 ml of the diluted soil suspension in 3 TSM agar plates for each sample and incubated at 25°C for 5 days. The strains were purified on TSM agar plates using sub-culture technique.
Pseudomonas fluorescence were isolated by serial dilution method using King’s B agar (Peptone: 20g/l, K2HPO4: 1.5 g/l, MgSO4: 1.5 g/l, glycerol: 10 ml/l, agar: 20g/l)28. 1 gm of each soil sample were suspended in 9 ml of sterile distilled water and vortexed (Accumax India, New Delhi-110058, India) for 5 min. The soil suspension was then serially diluted to 10-6. Pour plate technique was used by mixing 1 ml of the diluted soil suspension in 3 King’s B agar plates for each sample and incubated at 27°C for 48 h. The strains were purified on King’s B agar plates using sub-culture technique.
Bacillus subtilis was isolated by serial dilution method using Nutrient Agar (Peptic digest of animal tissue: 5 g/l, NaCl: 5 g/l, beef extract: 1.5 g/l, yeast extract: 1.5 g/l, agar: 20g/l)29. 1 gm of each soil sample were suspended in 9 ml of sterile distilled water and vortexed (Accumax India, New Delhi-110058, India) for 5 min. The soil suspension was then serially diluted to 10-6. Pour plate technique was used by mixing 1 ml of the diluted soil suspension in 3 NA plates for each sample and incubated at 27°C for 48 h. The strains were purified on NA agar plates by using sub-culture technique.
All thirteen isolates were screened against R. solanacearum by agar well diffusion technique30. R. solanacearum on CPG agar plates were transferred to nutrient broth and shaken in a rotary shaker (Talboys, Henry Troemner, LLC, USA) at 100 rpm at 27°C for 24 h. Similarly, the TSM, King’ B and NB were prepared for all Trichoderma spp., P. fluorescence and B. subtilis, respectively, and incubated for 7 days, 48 h and 48h, respectively. After incubation of the antagonists, 5 ml of broth suspension were centrifuged at 5000 rpm for 5 min and the supernatant was stored at 4°C for further procedure. Then, R. solanacearum suspension of 108 cfu/ml was prepared as per McFarland 0.5 turbidity method31 and was swabbed on NA plates. Holes of 5 mm were punched into the agar plate and 40 µl of the supernatant prepared were added separately and the plates were incubated at 27°C for 48 h. Inhibition of R. solanacearum growth was assessed by measuring the radius in mm of the zone of inhibition (ZOI) after incubation.
For microscopic visualization of the inhibition, CPG agar plates were prepared to provide the most favorable growth to R. solanacearum, and the respective 5 mm mycelial discs of Trichoderma species were added in the center of the plate after cotton swabbing from the CPG broth of R. solanacearum. For B. subtilis and P. fluorescence, the line was streaked parallel to the streak of R. solanacearum in two different CPG agar plates using dual culture technique. After 72 hr of incubation, live microscopic examination on the culture plate was done using a digital microscope (Olympus CX-43, Tokyo, Japan). Images were captured to visualize the interaction of the individual strains of biocontrol agents with R. solanacearum.
There is common practice in Nepal of keeping bio-based products in 5–10% (w/v) sucrose solution for 2–4 h before application to the plant. Hence, to evaluate the effect of 5% (w/v) sucrose solution on the cell number (growth) of the biocontrol agents, 1 ml of concentrate containing 1 × 109 cells ml-1 was kept in 5% sucrose solution, made using autoclaved distilled water. Cell count was taken using a Hemocytometer (Reichert, Buffallo, NY, USA) with trypan blue at 1 h, 2 h, 3 h and 24 h to observe the effect on microbial population.
For the field study, the concentrates containing 109 cells/ml of the respective biocontrol agent were used. The densities of the cells were determined using Hemocytometer (Reichert, Buffallo, NY, USA)32. The TSM broth of all six isolated native Trichoderma species viz. AA2, AG3, AKD, A5, A9 and A10 were mixed in equal proportion to prepare 1 liter of concentrate containing 109 cells/ml. Similarly, two native P. fluorescence species viz. PFB and PFS were mixed in an equal proportion to prepare 1 liter of concentrate containing 109 cells/ml. Concentrate of native B. subtilis viz. BS containing 109 cells/ml was used to analyze the effect of B. subtilis as a possible biocontrol agent.
Before the application in the tomato plots at Agro Narayani Farm, the prepared concentrate of biocontrol agents were taken to the field and were further diluted at the rate of 2ml/l of tap water containing 5% (w/v) sucrose. After 2 h of incubation in 5% sucrose water, the diluted solutions were applied in the root of tomato plants at the rate of 100 ml per plant. The processes of applications were repeated every 7 days for 8 weeks (total of 8 applications) by preparing fresh dilutions in 5% sucrose solutions 2 h prior to applications. Effects after the 8 weeks of continuous application were measured in the field by identifying the number of plants that underwent recovery after treatment. 6 plots were treated with the biocontrol agent and 2 plots were used as controls. Chemical treatment was done in one plot (positive control plot) using the combination of Agricin (9% Streptomycin Sulphate and 1% Tetracycline Hydrochloride) at the rate of 100 ml of 0.1% (w/v) solution per plant from Agricare Nepal Pvt. Ltd., Nepal and Bavistin (50% carbendazim) at the rate of 0.2 % (w/v) solution per plant from Crystal Crop Protection Pvt. Ltd., India. For negative control no treatment methods were selected in one plot.
The treatment plots were designed such that the effects of the individual biocontrol agent and effects of combination treatment can be studied (Table 2).
One plot comprised of 100 tomato plants and 8 plots in total were studied; area of 50 m2 per plot.
Weather data of Chitwan District for temperature, humidity and rainfall were collected from www.worldweatheronline.com from March 2017 to July 2017 (see Table 3).
| Month | Maximum temperature °C | Minimum temperature °C | Average temperature °C | Rainfall (mm) | Cloud (%) | Humidity (%) |
|---|---|---|---|---|---|---|
| March | 26 | 11 | 21 | 42.3 | 16 | 47 |
| April | 32 | 17 | 27 | 184 | 8 | 43 |
| May | 32 | 19 | 28 | 319.5 | 13 | 54 |
| Jun | 32 | 21 | 28 | 536 | 24 | 71 |
| July | 30 | 21 | 27 | 791.8 | 49 | 84 |
Source: www.worldweatheronline.com
The bacterial wilt outbreak was reported in the late May, 2017, when the temperature and humidity level increased. Higher temperature and moisture favors the growth of R. solanacearum33
From the field examination of the tomato plants, observation revealed that the leaves were flaccid, adventitious roots started to appear on the stem and ooze appeared after dipping the stem in water. Also, field experts from KCC confirmed the presence of bacterial wilt infection, due to their years of experience in plant disease diagnosis.
Infected plant saps from six xylems showed similar bacterial colonies on CPG medium. All the colonies were similar to avirulent type, as the appearance was white or cream-colored, irregularly-round, fluidal, and opaque on CPG medium34,35. Gram staining and observation using a microscope showed that the bacteria were gram negative, rod shaped and non-spore forming, which further confirmed that the bacteria was R. solanacearum.
All 13 strains tested showed antagonistic effect against R. solanacearum, with inhibition zone radii ranging from 13 to 21.33 mm (Table 4). P. fluorescence PFS isolated from Parthenium rhizoplane soil was most potent compared to other P. fluorescence strains. Trichoderma virens ATV and Trichoderma harzianum ATH provided by TNAU were the least and most potent species. Among six natively isolated Trichoderma sp., AA2 isolated from Parthenium rhizoplane soil was most potent and AKD and A9 were least potent. However, the activities of native Trichoderma spp. were satisfactory in the term of inhibition zone shown. Bacillus subtilis BS isolated from Bhaktapur top soil did not show satisfactory inhibition activity.
All the data are generated using three replications. Values are means (±SE) zone of inhibition (ZOI) in mm against R. solanacearum (n=3, P <0.05). S30 denotes streptomycin sulphate used at the dilution of 30 mcg. 5 mm diameter of punch hole is included in the data. Code is in reference to Table 1.
The effect of biocontrol agents against R. solanacearum was analyzed at a microscopic level in dark phase using image analyzer (Olympus CX-43, Tokyo, Japan). Figure 1–Figure 3 show the distinct inhibitory effect on the growth and survival of R. solanacearum caused by different biocontrol agents. From the figures it can be seen that the most of the pathogenic cells (R. solanacearum) were either killed or growth was retarded or limited in or towards the region of growth of antagonists as compared to region away from the growth of antagonists.
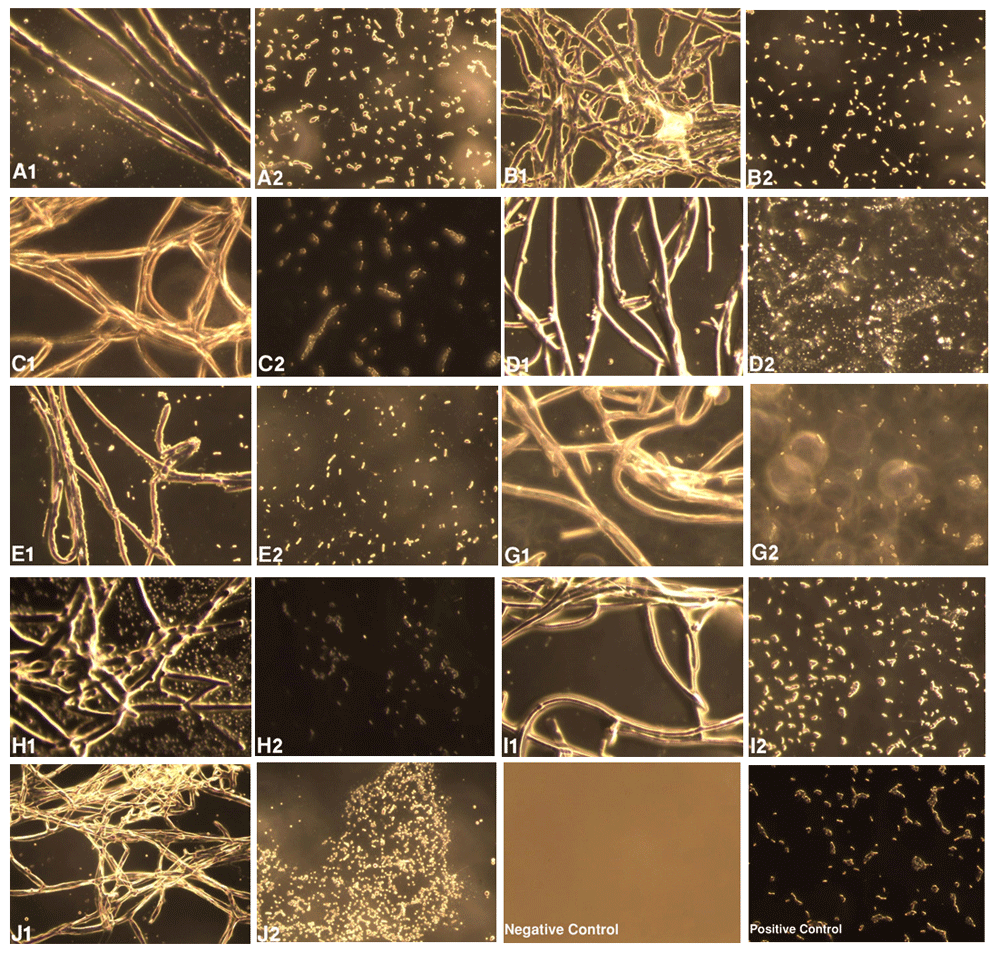
A1–J1 shows strains AA2, AG3, AKD, A5, A9, A10, ATA, ATH and ATV, respectively (see Table 1), growing on R. solanacearum, which was cotton swabbed onto CPG agar plates. A2–J2 represents growth of R. solanacearum 4 cm away from the growth of Trichoderma spp., viz., A2, AG3, AKD, A5, A9, A10, ATA, ATH and ATV, respectively, on the plate. Negative control (i.e., blank plate without any swabbing) and positive controls (i.e. R. solanacearum without Trichoderma species) are also included. The images reveal that the population of R. solanacearum is significantly less and most of the cells are dead in the region of growth of samples treated with Trichoderma spp., compared to the region 4 cm far from the growth of Trichoderma spp.
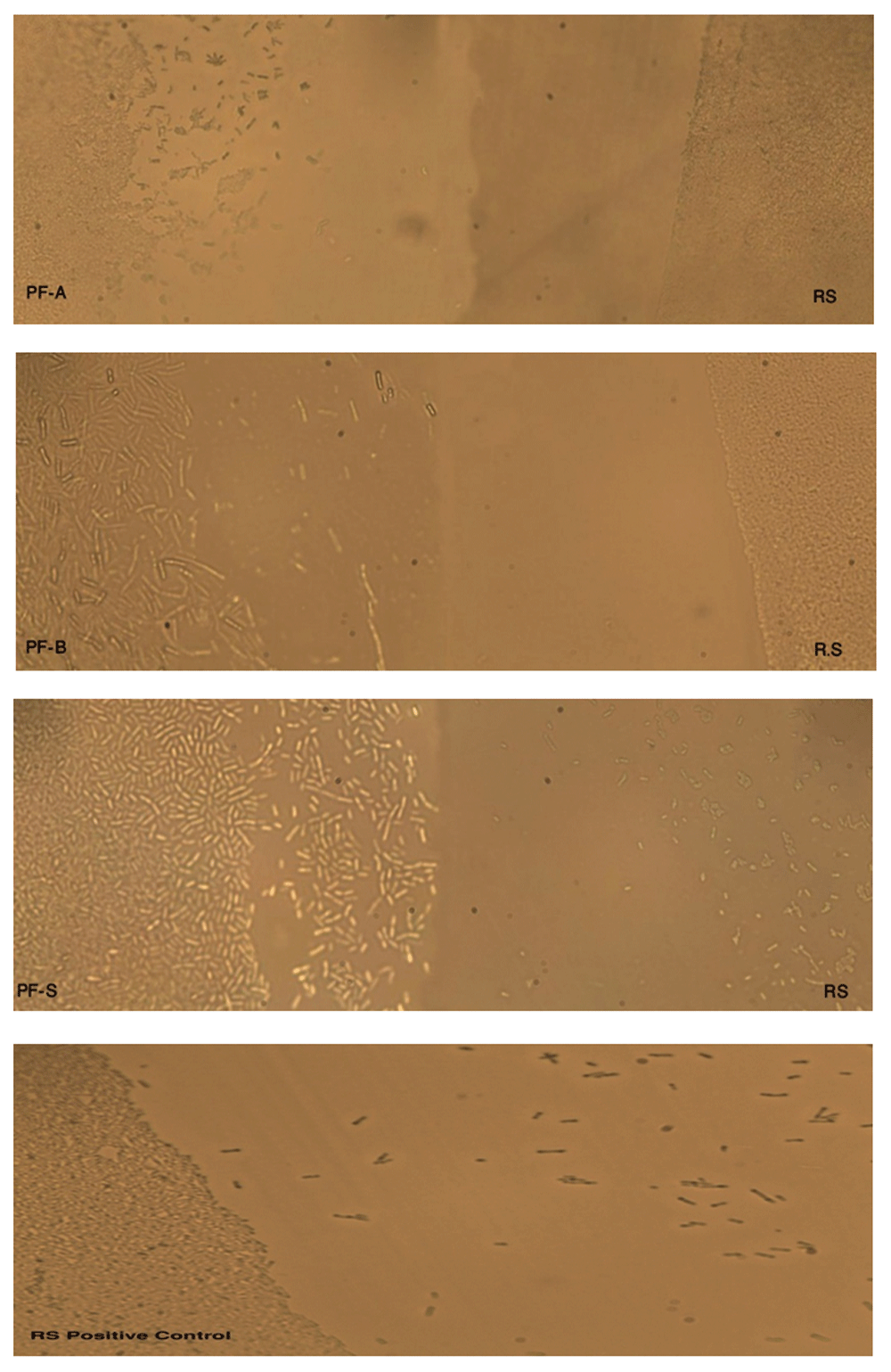
PFA, PFB and PFS represent P. fluorescence (PF) species (see Table 1), and RS represents R. solanacearum. Both PF and RS were streaked near to each other to see the interaction between the two species. PF tended to grow on the side of RS, whereas RS tended to restrict the growth towards the PF species. RS positive control (without PF streak nearby) tended to spread, which confirms the spreading pattern of RS.
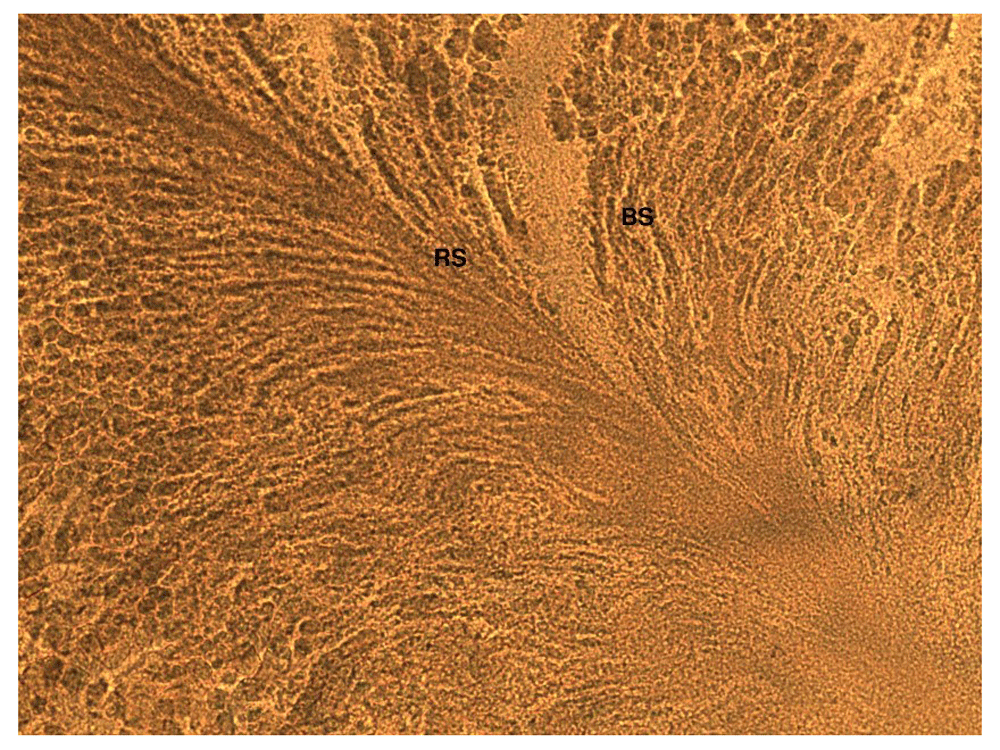
BS represents Bacillus subtilis species (see Table 1), and RS represents Ralstonia solanacearum. Both BS and RS were streaked near to each other to see the interaction between two species.BS has completely overgrown the RS streak on the CPG agar plate, which suggests that there has been an interaction between RS and BS.
The effect of sucrose (5% w/v) in the concentrate mixture of individual biocontrol agents was analyzed (Figure 4), which showed that there was profound increase in the number of cells of biocontrol agents after 2h of incubation compared with the initial population. The cell count between 2h and 3 h of incubation was not significant as compared with 2 h and 24 h of incubation. Thus, 2 h of incubation in sucrose solution can be considered as optimal time, as lengthier time can result in growth of contaminant in the solution whilst using tap water in the field.
Before applications, 2ml of respective biocontrol agents of (109 cells/ml) were incubated in 5% (w/v) of sucrose solution and incubated for 2 hr. The prepared dilution was thus applied at the rate of 100 ml per plant in the root region every week. 8 applications were done over 8 weeks. 8 plots (100 plant/plot) were selected, out of which one plot was used as positive control/chemical treatment plot (Agricin+Bavistin) and one plot as negative control/zero treatment plot (no treatment given).
The results are displayed in Table 5 and show that the application inhibited the bacterial wilt infection (R. solanacearum) in tomato plants by and the highest rate of plant recovered was 97% from treatment using antagonists, which was comparable with the plant recovery of 94% using the chemical treatment (Agricin and Bavistin). Only 37% of plants were recovered in the plot where no treatment methods were applied. Field Images (Figure 5) show a clear visualization of growth of plants and severity of infection in treated and untreated plot.
There was a significant recovery of plants using a mixture of Trichoderma species and Pseudomonas fluorescence, and the recovery rate was higher than that of chemical treatment. Bacillus subtilis did not show significant recovery rate.
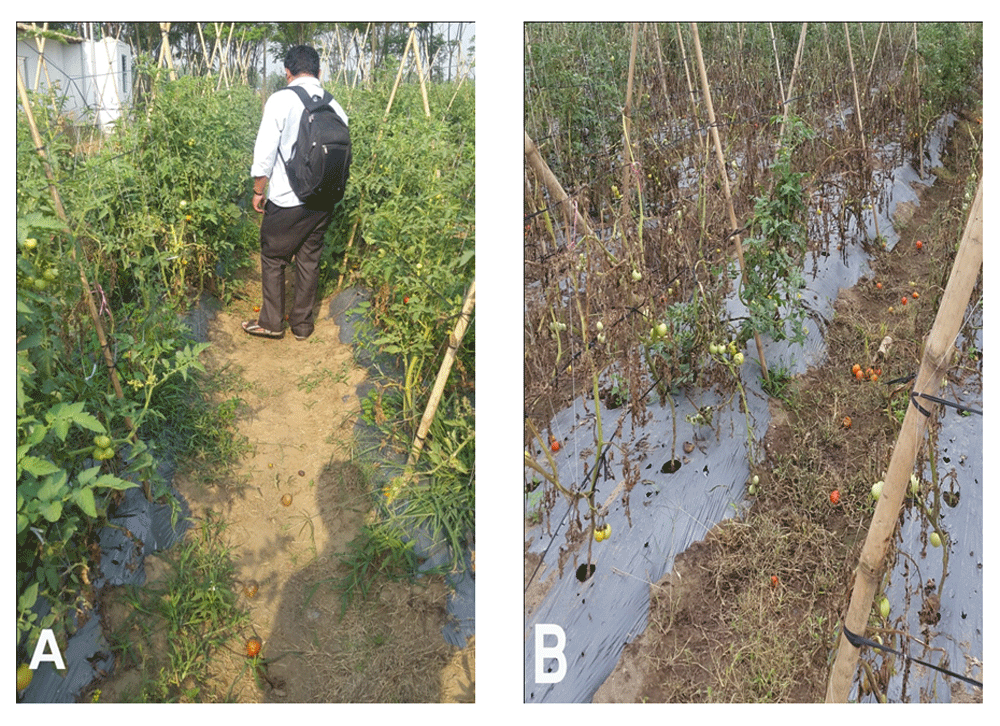
Field images clearly reveal that the treatment with biocontrol agents has helped to eliminate the bacterial wilt disease in the field after 8 weeks of application. (A) shows the growth and vigor of plants treated with biocontrol agents; (B) shows growth and severity of infection occurred in untreated plot.
This research covers the results of the effectiveness of native biocontrol agents in both laboratory and field settings, providing a complete portfolio from research in the lab to application in the field. This research also provides the application strategies of biocontrol agents at the field level from years of experience of expert field technicians. Also, from literature reviews10–17, it has been shown that these biocontrol agents can be used to control various other bacterial and fungal diseases, such as Fusarium wilt. Hence, application of these biocontrol agents can also help to prevent other diseases in various crops.
The present results, using microscopy, showed that different species of Trichoderma, Pseudomonas fluorescence and Bacillus subtilis clearly hinders the growth of Ralstonia solanacearum, which causes bacterial wilt in tomato plants. Trichoderma spp. secret different compounds against bacteria and also produce various secondary metabolites that promote plant growth and yield36–38. P. fluorescence produces various compounds that suppress the growth of R. solanacearum and also induces systemic resistance in the plant39–41. B. subtilis is well known to induce systemic resistance in plants by secreting various kinds of lipopeptides and secondary metabolites, and this agent also improves plant growth42–44. Analysis of data from research, field trials and scientific journal reviews suggests that the application of B. subtilis may not immediately show results, but continuous application of this strain in the agricultural field will slowly induce resistance of plants against pathogenic diseases42.
Pre-application of biocontrol agents can successfully prevent the disease attack45, induce systemic disease resistance in plants and increase the yield from secondary metabolites secreted by the beneficial bacteria in the biocontrol agent. Also, the farmers can sell their products by considering them as IPM (Integrated Pest Management) product or as an organic product, giving better monetary value for the farmers.
From the results, 2 h incubation of the biocontrol agent in 5% (w/v) sucrose solution was judged as suitable practice being carried out in Nepal for application of biocontrol agents, as it was seen that number of cells of biocontrol agents were increased during the incubation period. In addition, the water they use for drip irrigation generally is unsterilized and comes from an underground source, which may promote the growth of contaminants if kept for longer periods.
In the present study, Trichoderma spp. and P. fluorescence seem to be the best biocontrol agents in controlling bacterial wilt induced by R. solanacearum. The zone of inhibition shown by the various antagonists reveals that native isolates were successful in inhibiting the growth of R. solanacearum. The digital microscopy also supports the antagonistic effects of the native isolates. Also, combination therapy using both Trichoderma spp. and P. fluorescence seems to be more effective than treatment using each individual biocontrol agent. A 97% control rate was achieved using combination treatment in the field.
Also during field application, mixing with 5% (w/v) of sucrose solution and keeping it for 2 h seems to be an effective strategy in better management of bacterial wilt, as it increased the population of the microbes as the biocontrol agent before the actual application. The application strategies of biocontrol agents with the rate of 100 ml per plant per week successfully recovered the plants from the attack of the pathogen. However, the application rate and amount of biocontrol agents can be varied according to disease severity. Also, the application of the multiple numbers of biocontrol agents can be performed to achieve better results. Trichoderma spp. and Pseudomonas fluorescence provide better results in controlling bacterial wilt in tomato. Bacillus subtilis did not perform well in the immediate control of disease. Data from Table 5 reveals that biocontrol agents can be used as the sole method to control bacterial wilt, and the use of chemical methods can be avoided in the field.
Hence, native isolates of Trichoderma spp. and Pseudomonas fluorescence can be used as biocontrol agents to control the bacterial wilt and combined application of these beneficial microbes as bioantagonist can give better results in controlling bacterial wilt infection by R. solanacearum.
OSF: Raw values of zone of inhibition by antagonist against Ralstonia solanacearum. ZOI is shown in mm and the data were used for statistical analysis. http://doi.org/10.17605/OSF.IO/9TQCE46
OSF: Raw values of the 5% sucrose treatment. The average of these values in cells/ml was taken to create the data in the manuscript. http://doi.org/10.17605/OSF.IO/Q8FVU47
Figshare: Images of the plates showing the zone of inhibition by different antagonists against Ralstonia solanacearum. Clear zone of inhibition obtained by agar well diffusion technique indicates the bioefficacy of the selected bioantagonists against R. solanacearum. https://doi.org/10.6084/m9.figshare.5562058.v248
Figshare: Raw digital images (400X) of microscopic analysis representing the interactions of different antagonist against Ralstonia solanacearum. Collection of raw images obtained from digital microscopy in both dark field and bright field microscopy at 400 X zoom. https://doi.org/10.6084/m9.figshare.5561968.v149
Figshare: Field Images of plot design showing pictures of before the treatment and effects after the treatment. There is significant decrease in the occurrence of disease for the treated plots whereas the bacterial wilt has severely affected in the untreated plot. https://doi.org/10.6084/m9.figshare.5562373.v250
Data are available under the terms of the Creative Commons Attribution 4.0 license (CC-BY 4.0).
SY was actively involved in the generating the data from the field where as RGC was actively involved in generating data from the laboratory. BRP was actively involved in the generating data from both the field study and the laboratory as well as writing the manuscript. All authors read and approved the final manuscript.
No competing interests were disclosed.
Author endorsement: Dr. Sushil Thapa confirms that the author has an appropriate level of expertise to conduct this research, and confirms that the submission is of an acceptable scientific standard. Dr. Sushil Thapa declares he has no competing interests. Affiliation: Texas A&M AgriLife Research, Amarillo, TX 79106, USA.
This research was funded as a part of public private partnership activities of Agricare Nepal Pvt. Ltd with Winrock International Nepal for USAID’s KISAN Project, the Presidential Feed the Future Initiative to develop bio-products locally in Nepal. The grant number was AID-367-C-13-00004 with sub-awardee DUNS number 557770037.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would first like to thank Ms. Mona Sharma, Public Private Partnership Manager, Winrock International, Nepal and USAID for providing moral and financial support to conduct this research. We like to thank Prof. Dr. Sevugapperumal Nakkeeran, Department of Plant Pathology, Tamil Nadu Agricultural University (TNAU), Coimbatore, India for his supportive ideas in the research. We would also like to thank Ms. Santoshi Sharma, Ms. Shushilata Sapkota and Ms. Sweta Shrestha, interns at Agricare Nepal Pvt. Ltd., Nepal, Mr. Sarkal Jyakhwo, Field Technician at Kishan Call Center, Nepal and Mr. Aashish Khanal, Production Officer at Agricare Nepal Pvt. Ltd., Nepal for their supportive efforts during the project. Lastly, we would like to thank Mr. Deepak Gurung, the tomato farmer in Sukranagar, Chitwan, Nepal for reporting the problem of bacterial wilt and providing his field for the plot design and the study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant and agriculture biotechnology, genetic diversity and plant tissue culture, organic farming
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 22 Mar 18 |
||
|
Version 2 (revision) 21 Feb 18 |
read | |
|
Version 1 20 Nov 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)