Keywords
immune-magnetic cell sorting, lipopolysaccharide sensitivity, CD14+ve monocytes
immune-magnetic cell sorting, lipopolysaccharide sensitivity, CD14+ve monocytes
In the second revision of the manuscript following changes have been made.
1. A couple of sentences have been rewritten in the Introduction section to bring clarity on 'how positive magnetic sorting of CD14 may not be expected to trigger any signal transduction pathways'.
2. A typo has been corrected the Results section; sub section “Degradation of microbeads”.
See the authors' detailed response to the review by Aniruddha Roy
See the authors' detailed response to the review by Nadejda Beliakova-Bethell
Magnetic sorting is a common technique used to obtain a highly pure population of cells of interest from a mixed population of cells, making use of microbead conjugated antibodies against the cell surface antigen. Positive sorting involves the tagging of cells with magnetic microbead conjugated antibodies, followed by isolation of the labeled cells by placing them in a magnetic field. After positive sorting, cells that have microbead conjugated antibodies on their surface can be conveniently analyzed using flow cytometry (Miltenyi et al., 1990; Pei et al., 1998). Negative sorting involves the labeling of all cells, except the cells of interest, by incubating them in a cocktail of magnetic microbead conjugated antibodies and subsequently removing them by placing them in a magnetic field.
Cluster of differentiation 14 (CD14) are specific markers used to identify monocytic populations, and they act as a coreceptors for LPS (Guha & Mackman, 2001). Since CD14 lacks a cytoplasmatic domain, the positive magnetic sorting of CD14 may not be expected to trigger any signal transduction pathways or alter its functionality. However it has been demonstrated that anti-CD14 antibody reduces LPS responsiveness of monocytes (Kim & Kim, 2014).
We investigated the immunophenotypic behaviour and molecular characteristics of monocytes after both positive and negative sorting, by analyzing their response and proliferation to stimuli like LPS. The biodegradation profile of the attached microbeads from the CD14+ cells was also investigated.
The investigation was approved (project serial number: IHEC/#52/10) by the Institutional human ethics committee of the National Institute of Immunology, New Delhi-67, India.
Experiments were performed at the National Institute of Immunology, New Delhi. 20 ml of peripheral blood was collected from five healthy volunteers aged between 25–30 years, after obtaining their written informed consent. Blood was collected more than once from some of the volunteers and there was a minimum gap of three months between two successive sample collections. The peripheral blood mononuclear cells (PBMCs) were isolated from blood by density gradient centrifugation using HiSepTM LSM 1077 (Himedia, Mumbai; India). The obtained PBMCs were washed thrice with Dulbecco’s phosphate buffered saline (Himedia, Mumbai; India) and counted using the trypan blue dye exclusion method with a hemocytometer (Rohem Industries Pvt Ltd, India).
Human blood derived monocytes were sorted using anti-human CD14 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), as per manufacturer’s protocol. Similarly, monocytes were isolated by negative sorting using the monocyte isolation Kit II (Miltenyi Biotec, Bergisch Gladbach; Germany) according to manufacturer’s protocol.
The positively sorted CD14 positive cells were re-suspended in RPMI 1640 medium (Himedia, Mumbai; India) supplemented with 10% fetal bovine serum (FBS) (Biological Industries, Beit-Haemek Israel) and 1X antibiotic-antimycotic solution containing streptomycin sulphate, penicillin and amphotericin-B (Himedia, Mumbai; India), and plated at a density of 4 × 106 cells per well in 6 well low adherence plates (Corning, Tewksbury; USA). The cells were periodically harvested by gentle scrapping and passed through a magnetic column. Cells with and without bound microbeads (obtained in the flowthrough) were counted using a hemocytometer (Rohem Industries Pvt Ltd, India).
Monocytes separated either by positive or negative selections were re-suspended in RPMI 1640 media supplemented with 10% FBS and 1X antibiotic-antimycotic solution. 1 million cells/ well were plated in a 24 well cell culture plate (Corning, Tewksbury, USA) and placed in a humidified CO2 incubator (ShelLab, Cornelius; USA) at 5% CO2/ 37°C for 24 hours. The cells were examined for adherence and thereafter stimulated with 1 ml complete RPMI media containing 500ng/ml of LPS (Sigma, St. Louis; USA). Fresh media containing 500ng/ml of LPS was replaced at each time point (8h, 16h, 24h) for supernatant collection.
The supernatants collected at various time points were analysed for the presence of pro- and anti-inflammatory cytokines; IL-8, IL-10, TGF-β1 and RANTES, using cytometric bead array (CBA) Soluble Protein Flex Set (BD Biosciences, San Jose; USA) as per manufacturer’s protocol. Equal volumes of five independent samples were pooled and undiluted samples were analysed. The data was recorded using BD FACSVerse (BD Biosciences, San Jose; USA) and was analysed using FCAP Array software v3.0 (BD Biosciences, San Jose; USA). The assay samples were appropriately diluted to match the detection range of the CBA kit.
The magnetic sorted monocytes were plated at a density of 1 million cells per well in a 24 well plate. After allowing the cells to adhere for 24 hours, RPMI 1640 media supplemented with 10% FBS and 1% antibiotic-antimycotic solution containing 500 ng/ml of LPS was added to the respective wells. The culture plates were then placed in Cell-IQ (CM Technologies, Tampere; Finland) and specific fields were focussed using 10X objective magnification. Time lapse microscopy was performed and analysed for 76 hours at 30 minutes interval using live cell imaging and software (Cell IQ Analyser, Finland).
Sorted monocytes incubated with LPS were examined for secretion of various pro- and anti-inflammatory cytokines. The levels of IL-8, IL-10, TGF-β1 and RANTES at different time points after positively and negatively isolated CD14+ monocytes were incubated with LPS were analyzed. The secretion of IL-8 was observed to be at its maximum at 8 hours in negatively sorted monocytes and at 16 hours in positively sorted monocytes (Figure 1A). The IL-8 level in negatively sorted CD14+ cells was 6 times higher than positively sorted CD14+ cells. A similar pattern was observed for secretion of RANTES (Figure 1C) and TGF-β1 (Figure 1D), though the differences were not very pronounced. It was of significance to observe the reversed pattern for an anti-inflammatory cytokine, IL-10 (Figure 1B).

Greater secretion of the pro-inflammatory cytokines in negatively sorted CD14+ monocytes upon activation was observed, during proliferation of activated monocytes. Figure 2 shows the variation in the average number of cells per field (487μm×364μm) for positively and negatively sorted cells. Further, the progression of proliferation is shown in Video V1 for negatively sorted cells and Video V2 for positively sorted cells. Alongside Figure 2, the videos show that negatively sorted cells proliferated rapidly and extensively upon activation, and the maximum number of cells was reached after 16 hours. However, there was no clearly defined time point of maximum number of cells reached by positively sorted cells.
To examine the degradation of microbeads, PBMCs were labeled with anti-human CD14 antibody conjugated microbeads and sorted under a magnetic field. The sorted cells were maintained in a culture and the numbers of cells with and without microbeads were counted via flow cytometric analysis. Results of three independent samples are shown in Figure 3.
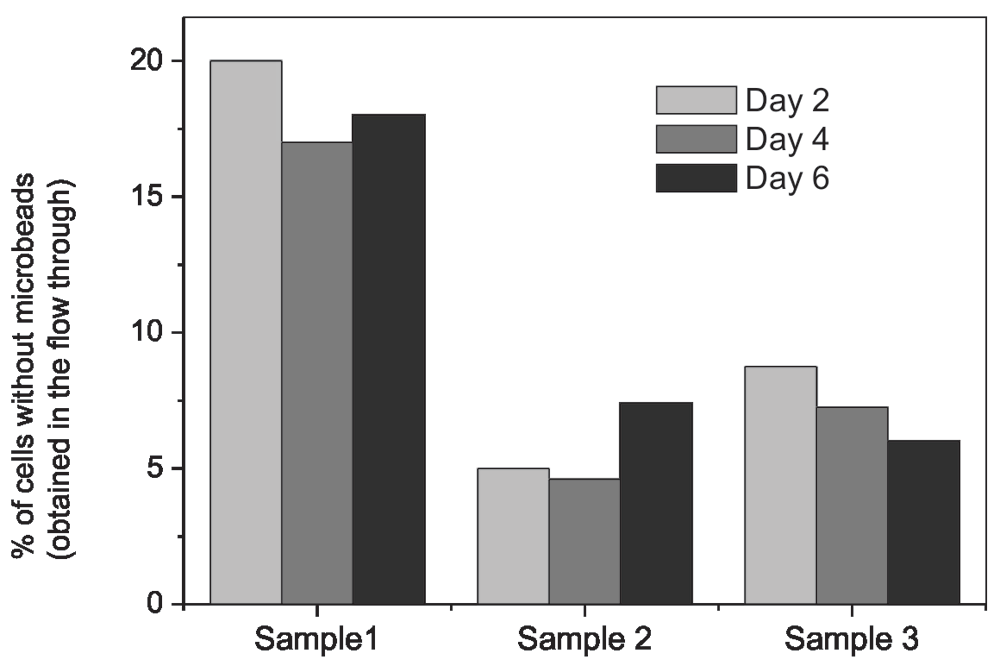
These results show the variation in percentage of cells collected in the ‘flow through’ of column placed in magnetic field. These were the cells from which the microbeads were either degraded or detached and cells were without microbeads. In all the three cultures we examined, the percentage of cells without microbeads was different but there was hardly any change in these percentages when the culture was continued for 6 days. This suggests that in typical culture conditions the Ab-microbeads remain bound with cells for many days.
| A | Day2 | Day4 | Day6 | E | F | G | H |
|---|---|---|---|---|---|---|---|
| Sample1 | 20 | 17 | 18 | ||||
| Sample 2 | 5 | 4.6 | 7.4 |
Magnetic cell sorting for the separation of large numbers of cells according to specific cell surface markers is a technique that is commonly used. It is a common notion that magnetic beads are biodegradable, do not activate cells and do not affect downstream application. We have however observed that the activation and proliferation of positively sorted CD14+ cells is impaired compared to the negatively sorted cells.
The activation of monocytes by LPS is known to occur through surface CD14, which is an LPS sensing receptor. Surface CD14 plays a crucial role; it binds and transfers LPS to the surface via TLR4:MD2 complex to enable its recognition. The LPS stimulation of monocytes activates several intracellular signaling pathways which in turn activates a variety of transcription factors ultimately leading to induction of many genes encoding inflammatory cytokines (Guha & Mackman, 2001). In short, CD14 is involved in the LPS-induced release of IL-8, which is an important pro-inflammatory cytokine (He et al., 2013).
After positively sorting CD14+ monocytes from PBMCs, the surface CD14 molecules on monocytes are blocked by anti-CD14 microbeads and these CD14+ surface sites can no longer mediate the stimulation by LPS and the positively sorted CD14+ monocytes may show impaired stimulation by LPS.
In two experiments, identical numbers of CD14+ monocytes isolated by positive and negative sorting were stimulated with LPS and their activation and proliferation was monitored. Figure 1 shows that upon stimulation by LPS the negatively sorted CD14+ monocytes secreted enormous amount of IL-8 almost instantaneously and they exhibited acute proliferation (Figure 2). This is in accordance with the observation that IL-8 transcript is highly expressed in LPS-stimulated monocytes (Standiford et al., 1992; Suzuki et al., 2000).
The positively sorted CD14+ monocytes responded only after 24 hours, and their level of stimulation was impaired and the cells did not proliferate. This delayed and reduced stimulation by LPS is due to the CD14 independent receptors which function to direct LPS mediated cytokine secretion under conditions where the CD14 dependent pathway is blocked or non-functional (Lynn et al., 1993). There are a few LPS-associated cell surface proteins which are distinct from CD14 and these surface proteins too can bind TLRs to initiate a response (Triantafilou et al., 2001). Our results suggest that the density of these surface proteins is low compared to CD14 as lesser stimulation was observed when CD14 surface groups were blocked by Abs and secondly, the delayed stimulation indicate that most likely a different pathway was followed for their activation.
Further, the secretion of somewhat higher amount of IL-10 by positively sorted CD14+ monocytes only suggest the absence of highly inflammatory conditions upon LPS activation. The levels of RANTES and TGF-β1 also indicate that the LPS activation of monocytes via CD14 independent receptors produces unique results which could be very different from common experimental situation.
In one such related report, the, human primary monocytes were isolated by either positive or negative immunomagnetic selection and differentiated to macrophages (Neu et al., 2013). The phagocytosis of Listeria monocytogenes (Lm) by GM-CSF-derived macrophages (GM-M) was markedly influenced by the method used for isolation of monocytes. The GM-M derived from negatively isolated monocytes showed low phagocytosis of Lm whereas GM-M generated from positively selected monocytes displayed high phagocytosis of Lm. The paper concludes that macrophages derived by ex vivo differentiation of negatively selected human primary monocytes as the most suitable model to study Lm infection of macrophages. In yet another report (Elkord et al., 2005) it was demonstrated that the human dendritic cells generated from positively isolated monocytes by anti-CD14-coated microbeads show impaired induction by LPS.
Beliakova-Bethell et al. (Beliakova-Bethell et al., 2014) examined the gene expression profiles of CD8+ T cells, B cells and monocytes isolated using positive selection, negative selection and FACS. They concluded that gene expression signatures were more similar between cells isolated by negative selection and FACS compared to cells isolated by positive selection. These findings were made on cells immediately after isolation and our findings establish the long-term effects of positive and negative isolation methods. In these reports it was not investigated further why the positively isolated monocytes were not suitable and had poor cytokines production upon stimulation. Our data suggests that in these experiments, the positively isolated monocytes were tagged permanently with anti-CD14 molecules attached with microbeads. The positively isolated CD14+ monocytes are identical with monocytes whose surface CD14 molecules have been blocked by Abs and these monocytes are known to behave differently (Delirezh et al., 2013; Elkord et al., 2005; Kim & Kim, 2014).
An alternate strategy to positively isolate monocytes could be by using anti-CD33 coated beads instead of anti-CD14 coated beads. The monocytes isolated using this approach too most likely will result in impaired response upon LPS stimulation. It has been reported that CD33 and CD14 are colocalized on cell surface and when monocyte-derived immature dendritic cells were stimulated with LPS in the presence of CD33 antibody, the production of IL-12 and phosphorylation of NF-κB decreased significantly (Ishida et al., 2014). The monocytes culture in the presence of anti-CD33 and the positively isolated monocytes using anti-CD33 coated beads, both have antibody bound CD33, and both are likely to respond in a similar manner.
In this context, diverse outcome could be observed when cells positively isolated by antibody bound microbeads were used for extended culture work (Govers et al., 2012; Greish et al., 2012; Lapenna et al., 2013; Meinhardt et al., 2012).
There are two important findings from these experiments; positively isolated CD14+ monocytes have impaired LPS sensitivity and magnetic beads used in positive isolation do not degrade within days. These conclusions suggest that for most experiments, positively isolated cells are usable for analysis purpose only and should not be used for any further culture experiments.
Dataset 1: Raw data corresponding to the results shown in Figure 1. DOI, 10.5256/f1000research.12802.d188477 (Bhattacharjee et al., 2017a)
Dataset 2: Raw data corresponding to the results shown in Figure 2. DOI, 10.5256/f1000research.12802.d182357 (Bhattacharjee et al., 2017b)
Dataset 3: Raw data corresponding to the results shown in Figure 3. DOI, 10.5256/f1000research.12802.d182358 (Bhattacharjee et al., 2017c)
This work has been supported by the core grant received from the Department of Biotechnology, Government of India, to the National Institute of Immunology, New Delhi.
This work was carried out at: National Institute of Immunology, New Delhi, India. The first author, Jashdeep Bhattacharjee, has now moved to: Division of Gastroenterology, Hepatology and Nutrition, Children’s Hospital Los Angeles.
Supplementary Video V1: The progression of proliferation after stimulation by LPS in negatively sorted CD14+ monocytes.
Click here to access the data.
Supplementary Video V2: The progression of proliferation after stimulation by LPS in positively sorted CD14+ monocytes.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Virology, immunology, cell separation and flow cytometry, analysis of gene expression
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 28 Mar 18 |
||
|
Version 2 (revision) 22 Dec 17 |
read | |
|
Version 1 23 Nov 17 |
read | |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)