Keywords
Caesarean section, obesity, intrathecal morphine, respiration, respiratory depression, apnoea/hypopnea
Caesarean section, obesity, intrathecal morphine, respiration, respiratory depression, apnoea/hypopnea
In caesarean section (CS), intrathecal morphine (ITM) is associated with better postoperative course with less pain and shorter time to mobilisation1. Respiratory depression associated with morphine is well known. Dahan et al. described the mechanism for the respiratory effects of opioids, defining the µ-receptor as the key target for the respiratory depressant effect and the sophisticated neuronal interaction in the ventral part of the brain stem2. Respiratory depression is the most feared adverse effect from intrathecal opioids, and although rare (0–0.9%), it is the reason for some anaesthesia units to withhold the addition of morphine to spinal anaesthesia for CS3–5. It has been suggested that ITM in doses <0.3 mg (300 µg) is associated with a lower risk of respiratory depression compared with doses >0.3 mg, and a tendency to a lower risk as compared to systemic opioids3,6. Palmer et al. found no analgesic benefit to exceed a 100 µg dose of intrathecal morphine, since incidence and severity of side effects increased1.
Respiratory depression is, however, not well defined and there is still not a general definition of respiratory depression to opioids7. George et al. conducted a meta-analysis on the primary outcome risk of respiratory depression and commented that multiple definitions were used in the included studies8. Similarly, a review by Ko et al. concerning the “definitions of respiratory depression”, explicitly addressing ITM, found no common description9. They also clearly commented the need for further research for defining what is a clinically significant respiratory impairment caused by ITM and how it best can be monitored9. Shapiro et al. defined is solely as a respiratory rate (RR) of <10 breaths per minute10.
Breathing disturbances postoperatively may be caused by several different factors. Residual effects from anaesthesia, abdominal surgery, obesity, immobilisation and opioid analgesics may all contribute to its occurrence7,11. Postoperative respiratory depression after CS, performed in spinal anaesthesia including neuroaxial morphine, is found in low frequency (0/5036, 8/856, 6/1915)4,12,13. Studies explicitly assessing the risk of respiratory compromise in obese patients, i.e. mothers with a high BMI (Body Mass Index), have not been previously conducted; apart from a retrospective study of 5036 mothers who had a CS with neuroaxial morphine, where 63% of patients were obese (BMI > 30 kg/m2), and the most commonly used morphine dose was 3 mg epidural and 0.15 mg spinal4. Crowgey et al. found no respiratory event with need for naloxone, defined as RR ≤8 breaths/min, oxygen saturation <90% or Richmond Agitation Sedation Scale <-2. Aboulish et al. published in 1991 the results from a study on ITM12. They found 8/856 cases of respiratory depression in women having intrathecal addition of 0.2 mg morphine in spinal analgesia for CS, all eight cases were obese and naloxone treatment did not reverse the respiratory depression during sleep12. Carvalho published a review in 2008 addressing the risk, monitoring and prevention of respiratory depression associated with neuroaxial morphine in the obstetric setting3. He summaries the risk and supports the need for observation up to 24 hours following neuraxial morphine, due to the duration of the depressed CO2 sensitivity. In addition, Carvalho commented on the present lack of efficient, simple and mother-friendly monitoring equipment; he supports RR, saturation and sedation monitoring3.
Portable sleep test equipment and at-home polygraphy monitoring is commonly used for screening for obstructive sleep apnoea. This equipment assesses respiration, actual gas flow, and saturation, and transforms the information into indices: apnoea-hypopnea index (AHI) and oxygen desaturation index (ODI) using defined algorithms.
The aim of this observational study was to explore the use of portable respiratory polygraphy for monitoring of obese mothers for respiratory depression over the first night after CS with bupivacaine/morphine/fentanyl spinal anaesthesia.
The study was approved by the Regional Ethical Review Board in Stockholm (2015/1257-31/2).
A prospective postoperative observational study was conducted from December 2015 to October 2017. Parturients with BMI >30 kg/m2 at the first antenatal consultation, who planned for elective CS with low transverse incision performed using standard spinal anaesthesia, were included in the study by the anaesthetist, after informed verbal and written consent was obtained.
Patients with language difficulties and known diagnosed obstructive sleep apnoea (OSA) receiving treatment, as those with continuous positive airway pressure or mouth guard, or any known contraindication to ITM were excluded.
Patients were informed at the preanaesthetic consultation about spinal analgesia and that they would receive a mixture of local anaesthesia, bupivacaine, and two opioids, fentanyl and morphine, in order to optimize perioperative and postoperative anaesthesia, according to the standard routine at our department (Anaesthesia & Intensive Care Unit, Danderyds Hospital).
Spinal anaesthesia was performed with the patient in sitting or left lateral position, at the anaesthetists’ preference, using a 25-gauge pencil-point needle. All patients received a mixture of heavy bupivacaine (11–12 mg), fentanyl (10 µg) and morphine (100 µg), according to standard routines for CS in our department. Preoperatively the patients received 1.5 g paracetamol orally, and paracetamol was continued postoperatively 1 g every six hours or 1.330g every eight hours. Ibuprofen (400 mg) was administered every eight hours. Additive medication to treat side effects, such as pain if numeric rating scale more than 3, nausea and vomiting or pruritus was administered according to the department's routines.
Routine monitoring in the postoperative and obstetric ward after spinal anaesthesia includes the following: checking for sedation, and if sedated counting RR every hour; pain by NRS/VAS; heart rate and blood pressure; control of bleeding; urine output; mobilization; and breast feeding. First mobilization, to stand by the bed, is usually encouraged at about 5–6 hours postoperatively. Urine catheter is normally removed after the first postoperative night.
Patients were informed at the preanaesthetic consultation about extended postoperative monitoring in addition to routine monitoring: nasal catheter to measure expiration flow; finger probe to measure oxygen saturation; thoracic and abdominal strings to collect breathing movements for polygraphy registration; a portable OSAS breathing pattern monitor, Embletta (ResMed Sweden AB, Kista, Sweden)/Nox Sleep monitor (Nox Medical, Iceland); and a combined ear-probe for transcutaneous carbon dioxide (TcCO2)/oxygen saturation (SpO2) monitor (Tosca Radiometer Medical ApS, Denmark).
Apnoea was classified in accordance to the American Academy of Sleep Medicine (AASM) as a drop in the polygraphy peak signal excursion by ≥ 90% of pre-event baseline air-flow signal14. The breathing disturbance was classified as mild AHI 5–15, moderate 15–30 and severe >30. The duration of the ≥90% drop in sensor signal must be ≥10 seconds14. Hypopnea was classified by as a drop in the peak signal excursion by ≥30% of pre-event baseline14. The duration of the ≥30% drop in signal excursions must be ≥10 seconds14.
Night-time respiratory monitoring device, that is polygraphy registration and Tosca as described above, was applied during rest/sleep during the first postoperative evening and night. For the 3–5 first hours postpartum, the patients were continuously observed awake in the postoperative department.
All patients answered a standardised ESS (Epworth Sleepiness Scale) questionnaire at time of enrollment.
Data is presented as the mean and standard deviation; categorical data are presented as frequencies. The study is explorative and observational, thus no power analysis has been conducted. Differences has been studied with Student’s t-test for continuous variables and Chi-squared test for categorical data. P<0.05 was considered significant. Data was analysed with StatView (v1.04) for MAC.
Forty mothers were invited to participate: 27 mothers consented but four of them had an early emergency CS delivery due to contractions, thus 23 mothers were included, but polysomnography registration failed in 3 (see Figure 1). Therefore, 20 mothers were included in analysis.
In all 20 mothers, the mean age was 35 ± 5 (24–43) years, mean BMI was 35 ± 4 (30–42), and mean ESS 6 ± 3 (0–12). For the ESS grade, 5 mothers had an ESS of <5, 12 had an ESS score between 5 and 10, and 3 scored >10.
Mean bed time during the polygraphic registration was 585 minutes (378–818). The mean registered SpO2 was 94 ± 1.3 (91–96) and mean nadir SPO2 71 ± 10 (49–81). In total, 4 mothers had a mean SpO2 <94 (91–93).
Mean AHI was 6.6 ± 5.2 (0–18.2) and mean ODI was 4.4 ± 3 (0–10.3). A total of 11 mothers had “normal” (<5) AHI, 7 had an AHI between 5 and 15, and 2 had an AHI 15–30. No mothers had an AHI >30. The longest apnoea duration was (mean) 30 ± 27 seconds, and mean longest hypopnea duration 55 ± 25 seconds.
Mean saturation was 94% (91–96) and four mothers had mean saturation between 90 and 94%, but no had a mean SpO2 < 90%. Nadir saturation was in mean 71% (49 – 81). In total, 11 mothers had an ODI <5, 8 had ODI between 5 to 10, and 1 mother had an ODI of 10.3. The 2 high AHI (15.3 and 18.2) mothers did not show high ODI or signs of hypercapnia on the transcutaneous CO2 registration.
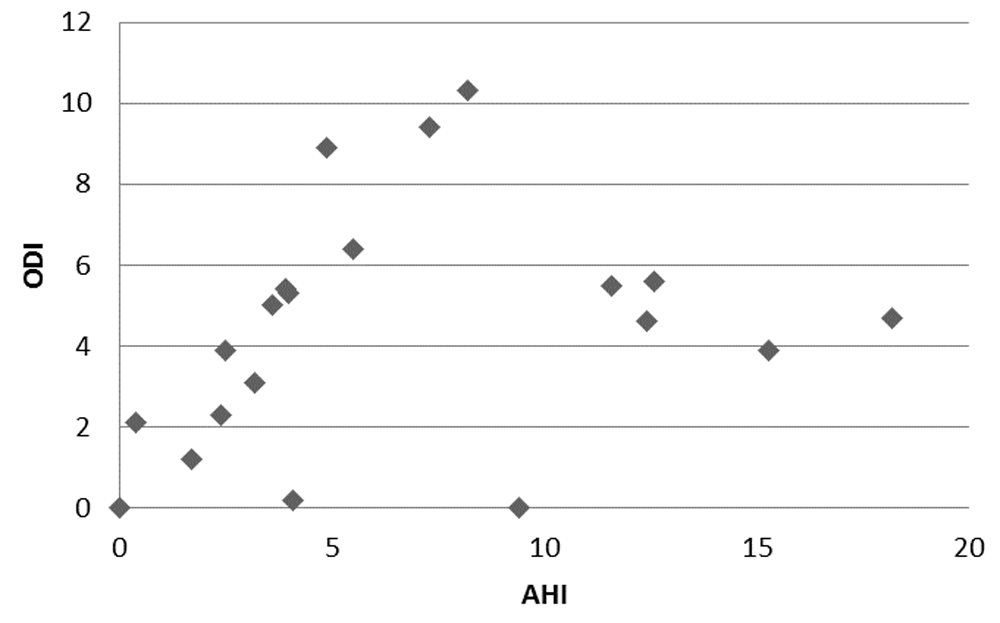
Mean TcCO2 was 4.7 ± 0.3 (4.1–5.2) kPa, and mean of max TcCO2 was 5 ± 0.5 kPa. There were no TcCO2 >5.9 kPa. The pattern between AHI, ODI, BMI and ESS was overall scattered without correlation; Figure 2 describes the AHI and ODI pattern.
None of the mothers showed clinical signs or symptoms of severe respiratory depression as assessed by routine clinical monitoring.
We found that two out of 20 mothers included in the present study had an AHI of >15 and none had an AHI defined as severe sleep apnoea. Mothers with a high AHI did not show typical high oxygen desaturation index or TcCO2 elevation. We did see frequent short episodes of oxygen saturation decrease, but we are unfortunately not able to assess whether these events were related to bradypnea or shallow breathing. We did not register any increase in TcCO2. The TcCO2 monitoring had a 15-minute averaging algorithm, thus it was not set for the detection of brief episodes of CO2 elevation. Respiratory depression typically progresses slowly3.
Studies with polygraphy registration sleep apnoea signs, associated with ITM are sparsely performed. The effects of 30 mg oral morphine on patients with mild to moderate obstructive sleep apnoea has been investigated previously by Wang et al.15. They found that morphine may paradoxically improve sleep apnoea15. Similarly, Bernards et al. studied the effects of remifentanil infusion, and found a decrease in obstructive events, but a worsening in oxygenation during the infusion16. Cole et al. studied the respiratory effects of ITM (dose, 300 µg) in prospective randomized fashion among patients having knee replacement in spinal anaesthesia17. They found similarly to the present study that night time respiratory polygraphy is cumbersome and associated with high patient non-compliance17. Among the patients studied in that study, the incidence of apnoea and hypopnea episodes was not significantly different compared to the control group of patients that did not receive ITM17. Median mean oxygen saturation was, however, significantly lower among the ITM patients and the occurrence of “mild and moderate hypoxia” was also high in the ITM group. In another previous study, 45 obese patients undergoing elective bariatric surgery with general anaesthesia were monitored in a similar fashion with portable polygraphy equipment during the first postoperative night on the general ward; only two patients with an AHI >5 and only three with an ODI >5 was found18. Therefore, our findings, that registration during the first postoperative night following CS with spinal anaesthesia with a modest dose of morphine in obese mothers did not show any high incidence of AHI and ODI, might not be that surprising. It is also in line with a Cochrane review assessing the effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea19. None of the studied drugs in the Cochrane review produced a significant increase in AHI or ODI and two trials have shown a beneficial effect on OSA19.
However, Subramani et al. describes, in a recent paper in the British Journal of Anaesthesia, catastrophic events in patients related to obesity and sleep apnoea, stating that ‘Morbid obesity, male sex, undiagnosed OSA, partially treated/untreated OSA, opioids, sedatives, and lack of monitoring are risk factors for death or near-death events’20.
We cannot further comment on how and how long obese patients having ITM should be monitored. It seems still of importance to monitor respiration in patients at risk. Monitoring of AHI and ODI seems, however, not to be of major help. It may be that simple RR monitoring, TcCO2 measure and SpO2 are more feasible techniques21,22. Kopka et al. suggest that TcCO2 may be more effective in detecting respiratory depression compared to SpO2 when patients receive supplementary oxygen23. Ladha et al. studied oximetry after CS having 150 µg morphine intrathecal24. They found frequent mild desaturation events and increased risk in patients with obesity. Bauchat et al. studied TcCO2 tension and found that hypercapnia events (>50 mm Hg for ≥2-minute duration) occurred frequently in women receiving 150 μg ITM for post-caesarean analgesia; higher baseline TcCO2 readings were observed in women who had hypercapnia events25. Dalchow et al. studied both TcCO2 and SpO2 and found more frequent changes, hypercapnia as compared to desaturation26. They concluded, ‘The incidence of opioid-induced respiratory depression detected by TOSCA is higher than previously reported by other monitoring methods. TOSCA may have a role in detecting subclinical respiratory depression in the obstetric population. Further studies with a control population are needed’26. Patients at risk should of course be assessed prior to surgery/anaesthesia. Optimal screening method is however not well defined11.
There are several limitations with our study. It is merely an observational study, and we could only include 23 mothers. We had a high number of mothers that declined to participate after having been informed about the monitoring techniques. The portable polygraphy is intended for use in the home for sleep apnoea screening instead of being in-hospital for a full polysomnography. The equipment involves straps around the thorax and abdomen, nasal prongs and a pulseoximetry probe all connected with cables to the monitoring unit. We included mothers with a BMI between 30 and 42 (mean 35) and none of our mothers had a known sleep apnoea. Merely three had an ESS of more than 10. Higher BMI and higher number of patients, possibly with more signs and symptoms of sleep apnoea would have been of interest. We are not able to assess sleep time, whether the mothers studied were asleep or merely rested. A full polysomnography would be needed for further in depth analysis. One may however strongly question whether that is ethical in a mothers’ first night after caesarean section.
In conclusion, we found in this explorative study that portable polygraphy is cumbersome and many mothers decline its use. It seems also reasonable to conclude that although episodes of oxygen saturation decrease were not infrequently noticed, upper airway collapse, obstructive hypo/apnoea, role as risk factor for respiratory depression during the first night after caesarean section in spinal anaesthesia with addition of low dose intrathecal morphine even in obese mothers seems minor. However, further studies with a combination of RR monitoring, TcCO2 monitor and SpO2 seems warranted, especially in high risk mothers. Preoperative screening in obese patients, at risk for sleep breathing disorder, is of course of value.
Dataset 1: Raw data for the study polygraphy on the first night after caesarean section in spinal anaesthesia with morphine in obese mothers by Hein et al. The three patients who were excluded from analysis are highlighted. doi, 10.5256/f1000research.13206.d18538827
This study has been supported by the Department of Anaesthesia, Danderyds Hospital. No external grants have been received.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 07 Feb 18 |
read | read |
|
Version 1 29 Nov 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)