Keywords
Patent foramen ovale, PFO closure, stroke, medical therapy
Patent foramen ovale, PFO closure, stroke, medical therapy
Jenny Lai was responsible for addressing all of the reviewers' comments. She also conducted the literature search, data analysis and interpretation advised by the reviewers. This resulted in changes throughout the text and also the creation of a Supplementary Table 6, which focuses on the venous thromboembolic events. Accordingly the authorship list has been revised. All authors on the manuscript have agreed to the change and the updated manuscript and current authorship list in its present form.
See the authors' detailed response to the review by Rajkumar Doshi
See the authors' detailed response to the review by Bernhard Meier
The association between the presence of a patent foramen ovale (PFO) and cryptogenic stroke has been established by previous case-control studies1. However, whether PFO closure is effective in reducing stroke events when compared to medical therapy is controversial. Three randomized trials, the STARFlex Septal Closure in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a PFO (CLOSURE I)2, the Clinical Trial Comparing Percutaneous Closure of PFO Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism (PC)3 and the Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT)4, were conducted. All of these trials showed numerically fewer events in the primary intention-to-treat analysis, but this did not reach statistical significance. Recently, two trials have focused on this issue. Firstly, the PFO Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrent (CLOSE) trial evaluated PFO closure or anticoagulation against antiplatelet therapy, with a primary endpoint of fatal or non-fatal stroke5. Secondly, the Gore Helex septal occlude and antiplatelet medical management of reduction of recurrent stroke or imaging-confirmed transient ischemic attack in patients with PFO (REDUCE) trial compared PFO closure to antiplatelet therapy only, with a primary endpoint of ischemic stroke, new ischemic stroke or silent brain infarction, demonstrating significant reductions in these events compared to antiplatelet therapy6. Moreover, long-term data of the RESPECT trial were recently published7. Given these new findings, we conducted a systematic review and meta-analysis of these randomized trials to evaluate the benefits and complication rates in PFO closure versus medical therapy.
The meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA; a completed checklist can be found in Supplementary File 1). PubMed and Cochrane Library were searched for randomized trials that compared the efficacy in stroke prevention of PFO closure with that of medical therapy. The following search terms were used for PubMed: "patent foramen ovale" AND (stroke OR embolism) and "randomized" AND "Trial". For Cochrane Library, the following terms were used: "patent foramen ovale" AND "closure" AND (stroke OR embolism). The search period was from the beginning of the databases through to 16th September 2017, with no language restrictions.
The following inclusion criteria were applied: i) the design was a randomized trial in humans, ii) the study compared stroke outcomes for PFO closure versus medical therapy. Quality assessment of randomized controlled trials was performed using the Cochrane Risk Assessment Tool (Supplementary Figure 1 and Supplementary Figure 2).
Data from the different studies were entered in Microsoft Excel (2016 Version). All publications extracted from the search strategy were assessed for compliance with the inclusion criteria. In this meta-analysis, the extracted data elements consisted of: i) trial name; ii) follow-up duration; iii) quality score; and iv) characteristics of the population, including sample size, sex and age. Two reviewers (GT and MG) independently reviewed each included study and disagreements were resolved by adjudication with input from a third reviewer (TL).
The number of events for: i) primary endpoint, ii) stroke, iii) transient ischemic attack (TIA), iv) all-cause bleeding complications, v) gastrointestinal complications [bleeding, ulceration, ulcer perforation], vi) short-term AF or AFL, vii) long-term AF or AFL, viii) venous thromboembolism, were identified and extracted independently by each reviewer from each trial. The Comprehensive Meta-Analysis Software (Version 2) was used for subsequent meta-analyses and statistical analyses. The event rates (events per patient-year) were used to calculate rate ratios for each study, which were pooled in subsequent meta-analyses.
Heterogeneity across studies was assessed using the I2 statistic from the standard chi-square test, which describes the percentage of the variability in the effect estimates resulting from heterogeneity. I2 > 50% was considered to reflect significant statistical heterogeneity and in such cases the random-effects model was used. To identify the origin of the heterogeneity, sensitivity analysis excluding one study at a time. Funnel plots showing standard errors against the logarithms of the odds ratio were constructed. Begg and Mazumdar rank correlation test was used to test for publication bias and the Egger’s test were used to detect publication bias.
A flow diagram detailing the above search terms with inclusion and exclusion criteria is depicted in Figure 1. A total of 91 and 55 studies were retrieved from PubMed and Cochrane Library, respectively. Of these, six studies met our inclusion criteria3–8. These were based on the following trials: CLOSURE I, PC, CLOSE, RESPECT and REDUCE. The original publication on the RESPECT trial was excluded because an update on longer term results was recently published7. Therefore, a total of five studies were included in this meta-analysis2–6. The baseline characteristics of these studies are listed in Supplementary Table 1. This meta-analysis included 1829 patients in the PFO closure arm (mean age: 45.3 years; 54% male; mean follow-up duration 50 ± 30 months) and 1972 patients in the medical therapy arm (mean age: 45.1 years; 51% male; mean follow-up duration 50 ± 31 months). For the meta-analyses, event rates (events per patient-year) were extracted and used to calculate rate ratios. The results of the statistical treatment of this meta-analysis are detailed in Supplementary File 2, with those of sensitivity analyses by the leave-one-out method described in Supplementary Figure 3–Supplementary Figure 8. Funnel plots plotting standard errors against the logarithms of the risk ratios are shown in Supplementary Figure 9–Supplementary Figure 14. In all of the analyses, Begg and Mazumdar rank correlation test suggested no significant publication bias (P > 0.05) and the Egger’s test demonstrated no asymmetry (P > 0.05).
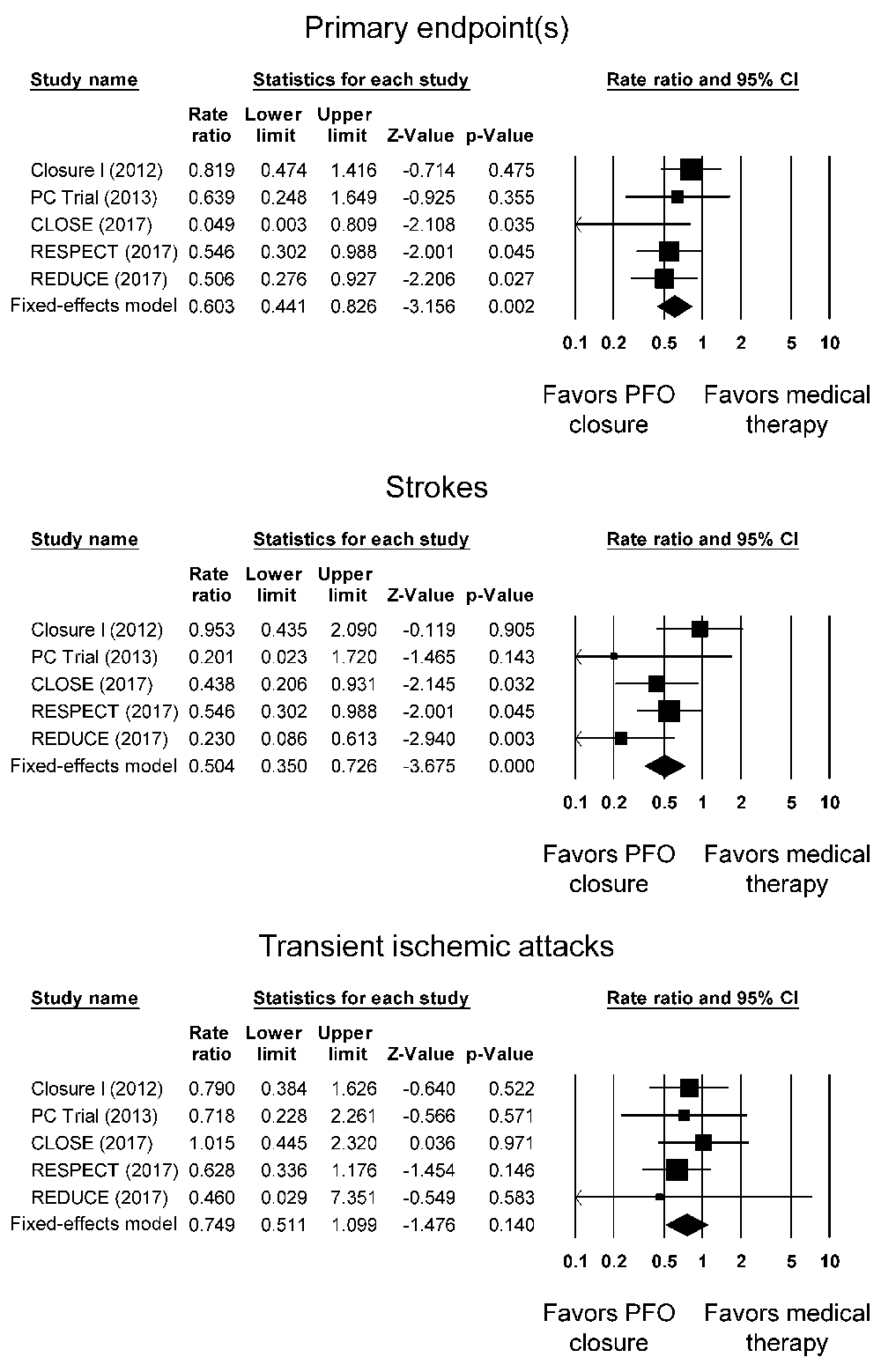
All five trials compared the primary endpoints in PFO closure versus medical therapy. The different trials used slightly different primary endpoints (Supplementary Table 2), as follows: 1) CLOSURE I trial: stroke, TIA, 30-day mortality, neurology-related death, 2) PC trial: stroke, TIA, death, peripheral embolism, 3) CLOSE trial: fatal or non-fatal stroke, 4) RESPECT trial: nonfatal ischemic stroke, fatal ischemic stroke, or early death after randomization and 5) REDUCE trial: co-primary endpoints of i) ischemic stroke, and ii) new ischemic stroke or silent brain infarction. Among the 1829 subjects who underwent PFO closure, 70 (3.8%) met the primary endpoint (Supplementary Table 3). By contrast, 112 of the 1972 subjects receiving medical therapy (5.7%) met the primary endpoint. Our meta-analysis shows that PFO closure significantly reduced primary endpoint events when compared to medical therapy with a rate ratio [RR] of 0.60 (95% CI: 0.44-0.83, P < 0.0001; I2: 15%) (Figure 2, top panel). Using hazard ratios [HR] from the trials produced negligible differences from our event rate analyses (HR: 0.61; 95% CI: 0.45-0.83, P < 0.01; I2: 0%).
Subgroup analyses were performed for the primary endpoint based on atrial septal aneurysm and shunt size by pooling hazard ratios from the subgroup analyses of the included studies. PFO closure was not significantly better than medical therapy for patients with an atrial septal aneurysm (HR: 0.46; 95% CI: 0.14-1.60, P = 0.22; I2: 62%, Supplementary Figure 15) or without an atrial septal aneurysm (HR: 0.74; 95% CI: 0.47-1.17, P = 0.19; I2: 0%, Supplementary Figure 16). By contrast, PFO closure significantly reduced primary endpoint events in patients with large shunt size (HR: 0.27, 95% CI: 0.14-0.54, P < 0.0001; I2: 0%, Supplementary Figure 17) but not in those with small shunt size (HR: 0.80, 95% CI: 0.49-1.31; P = 0.38; I2: 0%, Supplementary Figure 18)
All five trials compared the stroke events in PFO closure versus medical therapy. The different trials used slightly different primary endpoints, as follows: 1) CLOSURE I trial: acute focal neurological event that is magnet resonance imaging (MRI) positive, regardless of duration of clinical symptoms, or if imaging cannot be performed for confirmation, it was defined as a persistent focal neurological deficit lasting longer than 24 hours; 2) PC trial: any neurologic deficit lasting for >24 hours typically with documentation in MRI or computer tomography (CT); 3) CLOSE trial: sudden onset of focal neurological symptoms with the presence of cerebral infarction in the appropriate territory on brain imaging (CT or MRI), regardless of the duration of the symptoms (less than or greater than 24 hours); 4) RESPECT trial: ischemic stroke was defined as an acute focal neurologic deficit, which was presumed to be due to focal ischemia, and either symptoms that persisted for 24 hours or longer or symptoms that persisted for less than 24 hours but were associated with findings of a new, neuroanatomically relevant, cerebral infarct on MRI or CT; and 5) REDUCE trial: an acute focal neurologic deficit, presumably due to ischemia, that either resulted in clinical symptoms lasting 24 hours or more or was associated with evidence of relevant infarction on MRI or, if MRI could not be performed, CT of the brain. Taken together data from all five trials, stroke occurred in 45 patients (2.5%) in the PFO closure group, but in 102 patients (5.2%) in the medical therapy group. This gave a RR of 0.50 that was statistically significant (95% CI: 0.35-0.73, P < 0.0001; I2: 32%) (Figure 2, middle panel). Using HR from the trials produced negligible differences from our event rate analyses (HR: 0.49, 95% CI: 0.34-0.71, P < 0.01; I2: 30%).
TIAs were assessed in all five trials and the various definitions are shown in Supplementary Table 2. These occurred in 44 patients (2.4%) in the PFO closure group and in 67 patients (3.4%) of the medical therapy group (Supplementary Table 3). There was no statistically significant difference in the RR (0.75; 95% CI: 0.51-1.10, P = 0.14; I2: 0%) (Figure 2, bottom panel). Hazard ratios were available from four trials on TIAs, with a pooled HR of 0.73 (95% CI: 0.49-1.09, P = 0.13; I2: 0%).
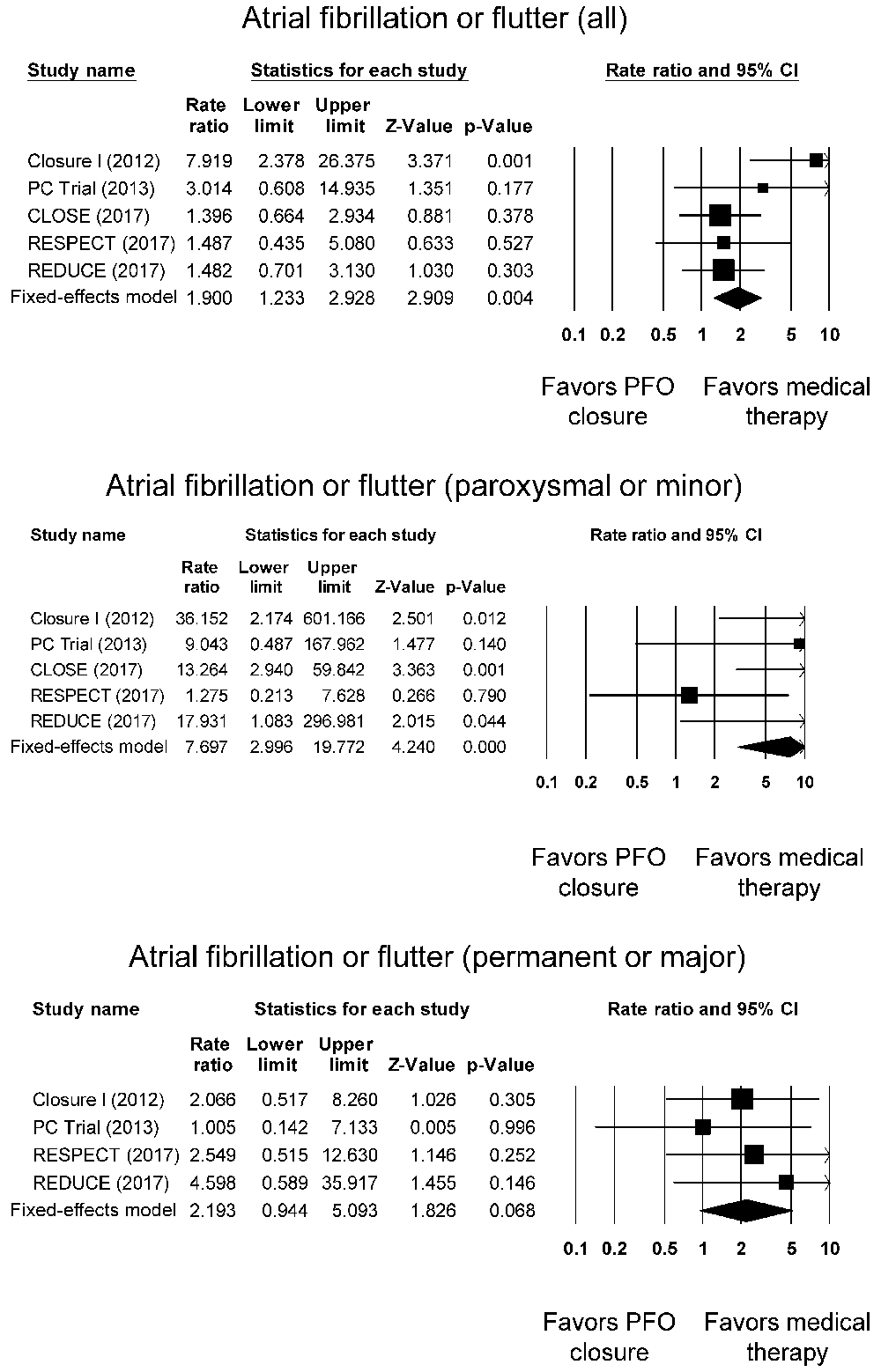
AF or AFL was detected in 76 patients in the PFO closure group (4.2%) and 37 patients (1.9%) in the medical therapy group (Supplementary Table 4). These equated to a significant increase in the risk when PFO closure was used (RR: 1.90, 95% CI: 1.23-2.93, P < 0.01; I2: 43%) (Figure 3, top panel). Subgroup analysis was performed for the type of AF or AFL by dividing the episodes into i) paroxysmal or minor, and ii) permanent or major [as defined by the individual trials]. This revealed that most of the episodes were only paroxysmal or minor for the PFO group (3.0%) when compared to the medical therapy group (0.6%) (RR: 7.70, 95% CI: 2.30-19.77; P < 0.0001; I2: 32%) (Figure 3, middle panel). Permanent or serious AF or AFL occurred in 1.3% in the PFO closure group compared to 0.4% in the medical therapy group without significance between these groups (RR: 2.19, 95% CI: 0.94-5.01; P = 0.07; I2: 0%) (Figure 3, bottom panel).
All bleeding complications were counted from the included studies (Supplementary Table 5). These were comparable between the groups, occurring in 39 (2.1%) of the PFO closure group and 47 (2.4%) in the medical therapy group, with no significant difference between them (RR: 0.89; 95% CI: 0.44-1.78, P = 0.74; I2: 51%) (Figure 4, top panel). Three trials reported gastrointestinal complications of hemorrhage, ulceration or ulcer perforation (Supplementary Table 5), which occurred in 7 and 8 patients in the PFO closure and medical therapy groups (0.4% for both arms). Therefore, the risk of gastrointestinal complications was not reduced by PFO closure (RR: 0.92; 95% CI: 0.32-2.70, P = 0.88; I2: 0%) (Figure 4, bottom panel). Venous thromboembolism, which comprised events of pulmonary embolism and deep venous thrombosis, were also counted from the included studies (Supplementary Table 6). Two trials, RESPECT and REDUCE trials, reported venous thromboembolism, which occurred in 20 and 6 patients in the PFO closure and medical therapy groups.
The key findings of this meta-analysis are that, compared to medical therapy, PFO closure significantly reduced primary endpoints by 40% and strokes by 50%, and had comparable risks of TIAs. Nevertheless, these benefits were observed despite a two-fold increase in the risk of AF or AFL in the PFO closure group. No difference in the risks of bleeding or gastrointestinal complications (bleeding, ulceration or ulcer perforation) was observed.
The foramen ovale remains open in about 25% of the healthy population, giving rise to a PFO9. PFO can be asymptomatic, but potentially causes cryptogenic strokes mainly through the mechanism of paradoxical embolization. PFO closure is used either for primary or secondary prevention of stroke. It has been proposed that PFO closure is an effective treatment to prevent recurrent stroke or TIA in patients with cryptogenic stroke if the shunt grade of the PFO is greater than moderate10. Long-term follow-up following percutaneous PFO closure for presumed paradoxical embolism have demonstrated very low recurrence rates11,12. Eustachian valve, Chiari’s network, medium-large shunt on trans-esophageal echocardiography, hypertension, age and the Essen stroke risk score have been associated with recurrent neurological events12–14. Medical treatment using antiplatelets or anticoagulants is an acceptable, alternative approach. A recent meta-analysis reported that anticoagulant therapy was more effective than antiplatelet therapy in preventing recurrent stroke and/or TIA, but with a 6-fold greater risk of major bleeding15.
The evidence from real-world studies has also been controversial. A small cohort including 159 patients <55 years old with cryptogenic stroke who received PFO closure or medical therapy did not show a statistically significant difference in the recurrence of ischemic events during a mean follow-up of 51.6 months16. In addition, in another small cohort including 164 patients with PFO and cryptogenic stroke the two groups (PFO closure vs. medical treatment) did not differ in regard to the composite end-point of death, stroke, TIA or peripheral embolism17. Similarly, data of the IPSYS registry, which included 521 patients aged 18–45 years old with cryptogenic stroke and PFO, showed no significant difference neither in composite end-point [ischemic stroke, TIA or peripheral embolism (P=0.285)] nor in brain ischemia (p=0.168) between PFO closure and medical treatment groups18. Additionally, Mirzada and colleagues did not find a difference regarding recurrent stroke or TIA between PFO closure and medical treatment groups19. Most of studies about PFO closure included young patients (<55 years old). A recent study, compared the outcomes of PFO closure between a young (<55 years old) and an old group (>55 years old) of patients20. It was found that in older patients, PFO closure was as safe as in younger patients, but recurrent cerebral ischemia was more frequent and likely this is associated to conditions related to age than to paradoxical embolism20.
Previous meta-analyses of randomized trials have found no statistically significant differences between PFO closure and medical therapy in the prevention of recurrent ischemic stroke21–24, whilst PFO closure was associated with an increased risk of AF21,24. By contrast, others have reported some benefits. For example, a patient-level meta-analysis reported that PFO closure reduced recurrent stroke and had a statistically significant effect on the composite of stroke, TIA, and mortality in adjusted analyses25. Moreover, another reported a 50% relative reduction of stroke and/or TIA versus antiplatelet therapy and by 82% relative reduction of major bleeding versus anticoagulant therapy15, whilst another reported that significant reductions in recurrent neurological events in intention-to-treat, per-protocol and as-treated cohorts26. A recent meta-analysis also reported that in comparison with medical treatment, PFO prevents recurrent stroke and TIA27. Further, another recent meta-analysis reported that in patients with PFO and cryptogenic stroke, transcatheter device closure decreases risk of recurrent stroke compared with medical therapy alone28. By contrast, anther meta-analysis reported that PFO reduced the risk of stroke, but not TIA, mortality, major bleeding and increased the risk of AF29. By pooling together data from two additional trials, our meta-analysis provides a firm conclusion that PFO closure produces a statistically significant reduction in the risk of not only primary endpoints, but also that of strokes. On subgroup analysis, we found that PFO closure significantly reduced primary endpoint events in patients with large shunt size, but not in those with small shunt size. Moreover, although PFO closure was no more effective in reducing TIAs when compared to medical therapy, what is important is that stroke, which is responsible for significant morbidity as well as mortality, was prevented. These benefits are also observed in real-world studies. For example, a long-term propensity score-matched comparison of PFO closure with medical therapy showed a mortality benefit30. These data also raise the issue regarding the benefit of primary prevention in cases with high-risk PFO31. Such a simple intervention might indeed be effective in preventing the first stroke event. As such, based on the results of our meta-analysis, it supports the need to primarily prevent high-risk PFO with PFO closure procedures instead of providing medical therapy. This approach is further by the increasing simplicity and success rates of the PFO closure procedure32. Despite the clear benefits, potential complications of PFO closure should be noted. For example, in the RESPECT trial, a significant increase in pulmonary emboli were observed (12 in the PFO closure group, of which 2 are listed as a complication of the procedure, vs. 3 in the control group) and deep venous thromboses (5 and 1, respectively). While the mechanism leading to the increased risk remains unclear, it may be possibly attributable to inappropriate application of groin compression subsequent to PFO intervention. Our meta-analysis also confirmed the increased risk of AF following PFO closure. Its clinical relevance is less certain for two reasons, that 70–90% of these events occurred in the first month and did not persist beyond this timeframe, and stroke incidence was reduced despite this AF occurrence. While the majority of AF occurrences were transient AF, it is not known how much subsequent monitoring these patients underwent or whether they were anticoagulated as a result.
In our meta-analysis of event rates for primary endpoint(s), low levels of heterogeneity were observed, which was probably due to the different definitions used across the trials. For example, two trials, CLOSURE I8 and PC3, included mortality as part of the primary endpoint, whereas the remaining three trials, CLOSE5, RESPECT4 and REDUCE6, included ischemic stroke events but not mortality. The medical therapy offered was similar across the studies, involving anticoagulation, antiplatelet therapy, or both. For the CLOSE trial, the investigators compared antiplatelet therapy with either anticoagulation therapy or PFO closure. For this particular trial, we had pooled data from both anticoagulation and antiplatelet therapy together, and compared the event rates with the PFO closure group. The present meta-analysis also demonstrates the safety of PFO closure when compared to more conservative medical therapy.
However, there are some limitations inherent to the trials themselves. Firstly, the crossover rate is substantial. For example, in REDUCE 6.3% of study subjects crossed over from PFO-closure to medical treatment and 6.3% did the opposite. Secondly, actual mechanical and anatomical closure of the PFO is of paramount importance to prevent recurrent paradoxical embolization. The different trials had different degrees of successful closure (e.g. REDUCE trial: 75%; CLOSURE trial: 86%; CLOSE trial: 93%) but these were not related to outcomes. Secondly, the definitions of TIA were different between the trials, which could have contributed to the between-study heterogeneity. Finally, the trials had large proportions of study subjects who were lost to follow-up, withdrew consent and crossed over to the other study arm, leading to uncertainty in the reported event rates.
There are many strengths of this meta-analysis study. It is the largest meta-analysis of randomized trials to date, including more than 3800 participants from five trials. Moreover, the follow-up duration was sufficiently long for events to be detected. Our quality analysis indicated that the studies had a low risk of bias. Low levels of heterogeneity were observed for most of our analyses, including primary endpoints, strokes, TIAs and gastrointestinal complications. These indicate that it was appropriate to pool these studies together. However, several limitations inherent in the present meta-analysis should be noted. Firstly, the meta-analysis of all-cause bleeding across the trials showed a high level of heterogeneity, which may be clinical in nature, especially when different types of bleeding were measured. Secondly, there is an imbalance between events and lost-to-follow-up/withdrawals33. In most trials, the ratio between the two is often in the region of 5 to 1, but is around 0.3 to 1 in the trials included in this meta-analysis. Moreover, publication bias results should be interpreted with caution as publication bias with less than ten studies is not recommended, but the results are presented here for the sake of completeness. Furthermore, there was a moderate degree of heterogeneity for the atrial septal aneurysm vs. no aneurysm comparison. Since our aim was to compare the effectiveness of PFO closure to medical treatment, we did not perform additional analysis by medical group assignment, such as comparing antiplatelet or anticoagulant therapy to PFO closure. The majority of medical management patients were treated with antiplatelet medications, with a minority treated with oral anticoagulation. Since the presumed mechanism of stroke attributable to PFO is paradoxical embolus from venous thrombi, or in situ thrombus formation, oral anticoagulants may be more effective for those causes. Where reported in individual trials, stroke outcome events were numerically less when medical management was with oral anticoagulation. Further analyses are needed to compare PFO closure with anticoagulation treatment alone as this was beyond the scope of the current study.
PFO closure significantly reduces the risk of primary endpoints, strokes, but not TIAs, when compared to medical treatment, despite higher rates of AF or AFL being observed. No differences in bleeding or gastrointestinal complications were detected between the two arms. PFO closure should therefore be considered for prevention of stroke in patients with PFO, especially in those presenting with cryptogenic stroke, who are less than 60 years of age with moderate to severe shunting.
All data required for reproducibility of this study are available from published studies.
GT and SW are supported by Clinical Assistant Professorship appointments by the Croucher Foundation of Hong Kong.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary File 1: PRISMA checklist.
Click here to access the data.
Supplementary File 2: Supplementary Figures 1–16.
Click here to access the data.
Supplementary File 3: Supplementary Tables 1–6.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Mechanical circulatory support devices, structural heart diseases, percutaneous coronary intervention, heart failure
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Shah R, Nayyar M, Jovin IS, Rashid A, et al.: Device Closure Versus Medical Therapy Alone for Patent Foramen Ovale in Patients With Cryptogenic Stroke: A Systematic Review and Meta-analysis.Ann Intern Med. 2018; 168 (5): 335-342 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Mechanical circulatory support devices, structural heart diseases, percutaneous coronary intervention, heart failure
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Lin M, Chen C, Hsu J, Lin H, et al.: Transcatheter Closure of Perimembranous Ventricular Septal Defects With Amplatzer Duct Occluders. JACC: Cardiovascular Interventions. 2017; 10 (21): 2227-2228 Publisher Full TextCompeting Interests: Speaker fees of Abbott.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 11 Sep 18 |
read | |
|
Version 1 27 Dec 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)