Keywords
MRI, PAX6, neuroanatomy
MRI, PAX6, neuroanatomy
We would like to thank our reviewers for their insightful comments and suggestions that contributed towards an improvement of the manuscript. Specific details of the changes can be found in our response to reviewers. Significant changes include: 1) amendments to the identification system for the patients to reflect the same numbering used in a previously published paper on this group of patients; 2) an added supplementary table (supplementary table 2) to allow for identification of each patient and their corresponding DICOM file; 3) addition of predicted protein effects for each mutation in Table 1; 4) the addition of a quantitative analysis of corpus callosum volume, total white and grey matter volume, and whole brain volume (supplementary table 1 and supplementary figure 1) for aniridia and PAX6-normal comparisons.
See the authors' detailed response to the review by Lutz Jäncke
See the authors' detailed response to the review by Veronica van Heyningen
Aniridia is panocular, congenital, and progressive disorder with an occurrence of approximately 1 in 83,000 live births1,2. Aniridia is best characterized by the lack of or hypoplasia of the iris (for which it is named), in addition to several other ocular abnormalities, which culminate in reduced visual acuity3. Due to the progressive nature of the disease, individuals usually develop multiple ocular abnormalities, such as keratopathy, corneal vascularization and opacification, glaucoma, anterior chamber fibrosis, and cataracts4–7. Although aniridia is most well known for its ocular phenotypes, the condition has a number of other abnormalities, including sensory, neural, cognitive, and auditory processing abnormalities8,9.
The development of aniridia in humans is linked to heterozygous loss-of-function mutations to the PAX6 gene, which encodes a highly conserved transcription factor critical for normal eye and neural development1. The vast majority of aniridia cases (80%) are associated with mutations in PAX62,10. Functional mutations in this gene can be either sporadic or familial, and causal mutations in aniridia encompass a large number of variants. The majority of these variants are nonsense mutations, which lead to a premature termination codon, and are found across the PAX6 locus10,11. PAX6 is expressed in the developing eye, brain, and spinal cord, and is required for aspects of anatomical and functional development of the central nervous system (CNS) and visual system12. Within the CNS, PAX6 is involved in patterning, regionalization, and the formation of neural circuits13–16. Previous studies of patients with aniridia using structural magnetic resonance imaging (MRI) have shown abnormalities in major fiber tracts and subcortical structures of the brain, including the anterior commissure9,17–20, posterior commissure18,20, corpus callosum9,19,20, pineal gland18,20, optic chiasm20, and olfactory bulb17,18. The most consistently reported abnormalities are found in the anterior commissure, pineal gland, and optic chiasm; abnormalities in the posterior commissure, corpus callosum, and olfactory bulb are found in fewer than 35% of patients examined. Additionally, studies have shown conflicting results regarding grey matter volume differences in aniridia, with reports of both increases and decreases in whole brain grey matter21,22. Most recently, it has been shown that there is an accelerated age-related increase in cortical thinning of the inferior parietal lobe and prefrontal/premotor areas in both brain hemispheres in aniridia compared to healthy patients23.
While several previous studies have investigated structural brain abnormalities in patients with aniridia, the variance in brain structures affected and extent of anatomical abnormalities is high. The variance observed in the published literature may be interpreted as a result of genetic differences in patient samples, either directly related to disease-causing mutations or modifier effects caused by genomic differences across subjects. Most of the previous studies examining structural changes in the brain of aniridia patients have focused on a subset of the structures we examined, but only one other study has looked at all five together20. Additionally, we used a 3T magnet instead of a 1.5T, which allows for higher resolution structural images of small structures such as interhemispheric commissures, allowing us to more reliably identify subtle difference. The current study sought to investigate gross anatomical correlates of aniridia in a new population sample with varied PAX6 mutations. Results from this study will serve as a comparison for previous studies, as well as contribute to what is known about the distribution of neuroanatomical phenotypes in the aniridia population as a whole. Overall, this will serve to clarify the extent of abnormalities in five brain structures in persons with aniridia with varied mutations to the PAX6 gene and contribute to our global understanding of the neuroanatomical characteristics of the disorder.
A total of 14 individuals with aniridia and 15 healthy comparison individuals participated in the current study. Data from two participants with aniridia were excluded from analyses (due to a significant artifact and missing data). One healthy subject was excluded due to an anatomical abnormality, and two others were excluded because they did not match the demographic profile of an individual in the aniridia group included in the analysis. The remaining 12 individuals with aniridia (7 females; 3 left handed; mean age=36 years, SD=15) and 12 age- and gender-matched comparisons (7 females; 4 left handed; mean age=35 years, SD=14) were included in the analyses (Table 1). Healthy comparison subjects were recruited through flyers posted in the community. Participants with aniridia were recruited through the Aniridia Foundation International Conference held in 2011 in Athens, Georgia and had been clinically diagnosed with aniridia. Exonic sequencing of the PAX6 gene (11p13) (OMIM: 607108) was conducted at the University of Georgia, as previously described10,24. All mutations, which can be found in Table 1, have been submitted to the Human PAX6 Allelic Variant Database (http://lsdb.hgu.mrc.ac.uk/home.php?select_db=PAX6), as part of a previous genotype identification study10. Three of the participants with aniridia belonged to the same family, and all other participants included in the analyses were unrelated. After written informed consent was obtained and MRI safety screening was conducted, all participants completed an MRI session in which a high-resolution structural scan was obtained. The Institutional Review Board of the University of Georgia approved all activities prior to subject recruitment, data collection, and data analysis (project number: 2011-10862-1; STUDY00003122).
Gender, age, handedness, mutation, and structural abnormalities of both aniridia subjects and healthy comparisons. Subject ID: Numbers are matched subjects, A refers to aniridia subjects, C refers to healthy comparisons. ND, not determined.
| ANIRIDIA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID | Gender | Age | Handedness | Mutation | Predicted Mutation Effect | Anterior Commissure | Posterior Commissure | Pineal Gland | Corpus Callosum | Optic Chiasm |
| 1A | Male | 18 | Right | c.949C>T | Nonsense | Reduced | Normal | Reduced | Normal | Reduced |
| 2A | Female | 19 | Ambidextrous | c.771delG | Frameshift deletion | Reduced | Normal | Reduced | Slightly reduced | Reduced |
| 3A* | Male | 20 | Right | c.204delC | Frameshift deletion | Reduced | Normal | Reduced | Normal | Reduced |
| 4A* | Female | 24 | Left | c.204delC | Frameshift deletion | Reduced | Reduced | Reduced | Normal | Reduced |
| 5A | Female | 25 | Left | ND | ND | Reduced | Reduced center | Highly reduced | Normal | Reduced |
| 6A | Female | 28 | Right | c.28C>T | Nonsense | Reduced | Normal | Absent | Slightly reduced | Normal |
| 7A | Male | 39 | Right | c.482delG | Frameshift deletion | Reduced | Normal | Reduced | Normal | Normal |
| 8A | Male | 47 | Right | ND | ND | Reduced | Normal | Reduced | Normal | Normal |
| 9A* | Male | 46 | Left | c.204delC | Frameshift deletion | Reduced | Normal | Reduced | Normal | Reduced |
| 10A | Female | 51 | Right | c.766-3C>G | Splice junction disruption | Reduced | Reduced | Absent | Normal | Normal |
| 11A | Female | 53 | Right | ND | ND | Reduced | Reduced | Reduced | Normal | Normal |
| 12A | Female | 60 | Ambidextrous | c.799A>T | Nonsense | Reduced | Reduced | Reduced | Slightly reduced | Reduced |
All data were collected on a 3T GE Signa MRI (General Electric, Milwaukee, WI, USA) at the University of Georgia’s Bio-Imaging Research Center. To obtain a high-resolution structural scan, images were acquired with a T1-weighted 3D FSPGR sequence [echo time (TE)=min full, flip angle=20°; field of view (FOV)=240 mm × 240 mm; matrix size=256 × 256, 150 axial slices; in-slice resolution=0.94 × 0.94 mm; slice thickness=1.2 mm].
MR images were transferred to a DICOM image format and analyzed using two software programs, SPM run on MATLAB and OsiriX. For SPM analysis, DICOM files were converted to nifti format and analyzed using Statistical Parametric Mapping Software (SPM8; Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/) run on a MATLAB software platform (MATLAB Release 2015b; The Mathworks, Inc., Natick, MA, USA). SPM software was used to compare aniridia subjects to their demographically matched comparison subjects. DICOM files were additionally analyzed using Osirix Lite DICOM viewer (OsiriX v5.6,;Pixmeo SARL, Bernex, Switzerland) and all images generated using this software. All 24 individual subjects’ data were visually inspected for gross anatomical abnormalities in two independent sessions by the first author (MG) and a radiologist (JDW). Regions of interest were determined by literature review to include the anterior commissure, posterior commissure, pineal gland, corpus callosum, and optic chiasm. The only structure that has been examined in the literature that we did not examine in our population was the olfactory bulb. This structure was excluded because both evaluators independently determined that the olfactory bulb could not be reliably assessed in the current data set. The radiologist was blinded to patient status and genotype during visual examination of scans. No new regions of interest were determined during both the first and second examination of the scans.
Following the approach of Free and colleagues (2003), the cross-sectional area of the corpus callosum was quantified at the mid-sagittal plane21. In order to align all MR images to the mid-sagittal plane, individual T1 images were reconstructed in AFNI software25 and aligned manually using the anterior commissure, posterior commissure, and interhemispheric landmarks. On the mid-sagittal image, the corpus callosum was manually traced and the number of voxels within this delineation was counted. Additionally, the structural volumes were automatically segmented with FreeSurfer software (http://surfer.nmr.mgh.harvard.edu) to obtain estimates of total grey and white matter volume26,27. The ratio of corpus callosum area to total cerebral volume was calculated to control for differences in overall brain size (see Supplementary Figure 1 “Ratio”). Supplementary Figure 1 graphs were created using GraphPad Prism 7 software (https://www.graphpad.com/).
Whole brain analysis was used to identify major structural abnormalities in aniridia patients. All neuroanatomical results are reported in Table 1, which includes subject demographics and mutations. We analyzed five major brain structures that demonstrate clear anatomical abnormalities in aniridia patients. Our study showed that all 12 aniridia patients had a reduced anterior commissure when compared to their demographically matched healthy comparisons, as shown in Figure 1. The posterior commissure was reduced in 5/12 aniridia patients and normal in 7/12 of aniridia patients (Figure 1). Of the five aniridia patients with reduced posterior commissures, one had a reduced commissure at the midline. The pineal gland was affected in all 12 aniridia patients: absent in two (Figure 1), highly reduced in one, and reduced in nine. The corpus callosum was slightly thinned in 3/12 aniridia patients and normal in 9/12 aniridia patients (Figure 2). The optic chiasm was reduced in 7/12 of patients (Figure 2) and normal in 5/12 patients.
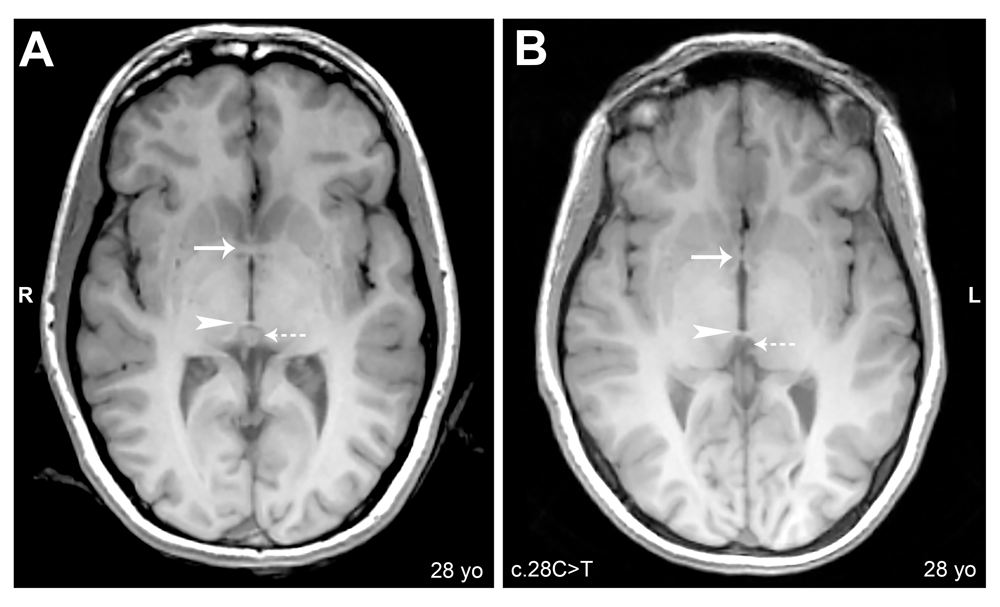
Axial cerebral T1-weighted magnetic resonance images, slice thickness 1.2mm. (A) Subject 6C: Arrow shows normal anterior commissure; arrowhead shows normal posterior commissure; dashed arrow shows normal pineal gland. (B) Subject 6A: Arrow shows reduced anterior commissure; arrowhead shows normal posterior commissure; dashed arrow shows absence of pineal gland.
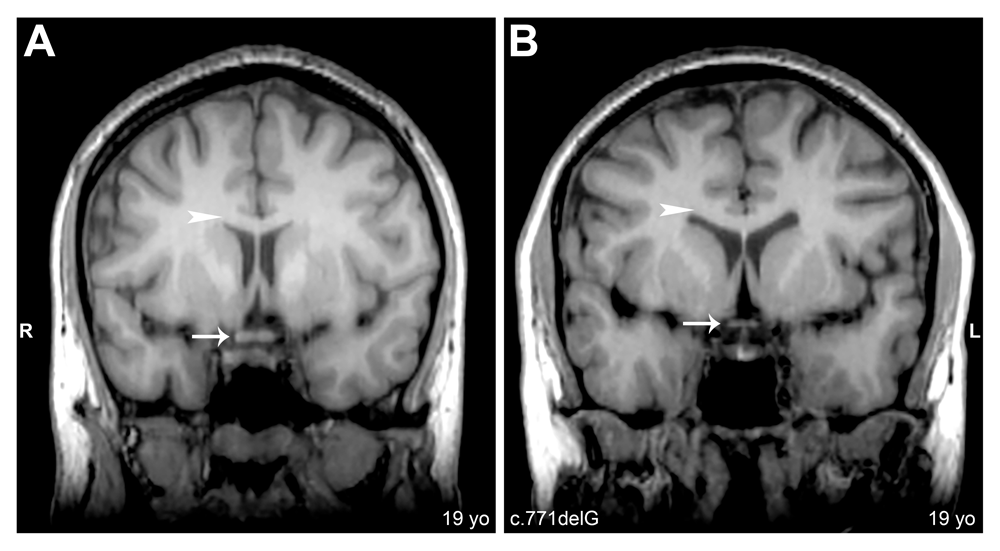
Coronal cerebral T1-weighted magnetic resonance images, slice thickness 1.2mm. (A) Subject 2C: Arrow shows normal optic chiasm. (B) Subject 2A: Arrow shows reduced optic chiasm. Arrowheads in A and B denote normal corpus callosum.
In an effort to consider normal structural variation in the healthy population, we also evaluated healthy comparisons for structural brain abnormalities. We saw a reduced anterior commissure in two of the healthy comparisons and a reduced posterior commissure in one healthy comparison. The pineal gland showed the most variance within the healthy comparison group with one subject with no visible pineal gland, three healthy comparisons with slightly reduced to reduced pineal glands, and eight healthy comparisons with normal pineal glands. All healthy comparisons had normal corpus callosums and optic chiasms. A full description of which structures showed abnormalities in both the aniridia and healthy comparison groups are reported in Table 1. These findings provide context for asserting disease-related changes in the aniridia population, in the current study as well as others.
Additionally, we examined corpus callosum area at the mid-sagittal plane along with grey matter, white matter, and whole brain volume in our aniridia and healthy comparison groups. Grey matter volume and total brain volume averages are indistinguishable between aniridia and healthy comparison subjects. There is a slight reduction in the aniridia patients compared to healthy individuals in average corpus callosum area as well as white matter volume and ratio of corpus callosum area to whole brain volume. However, these are just trends and no measurement was statistically significant, which can also be explained by a high degree of variability within the aniridia population. Volumetric and area measurements along with graphical representations of the data can be seen in Supplementary Table 1 and Supplementary Figure 1.
The most commonly reported neuroanatomical abnormality in MRI studies of aniridia patients is the anterior commissure. Previous studies have reported changes in the anterior commissure with some cases described as reductions and others reported as complete absence of the structure9,17–19. Consistent with these reports, our study identified a reduction in the anterior commissure in all 12 aniridia patients, but none of the patients lacked the structure. The posterior commissure has also been evaluated in previous studies: one study reports that it is present in all subjects while the other study presented evidence that one patient had an absent structure while the others had normal posterior commissures18,20. We found no individuals lacking the posterior commissure, and fewer than half of our aniridia patients seemed to have an abnormal structure. Interestingly though, it seems as if one patient exhibited a reduction of the posterior commissure at the midline with thickened bundles adjacent to the midline suggesting that commissure formation was incomplete. PAX6 has a known role in formation of the posterior commissure in rodents, so it is likely that this phenotype in humans is a direct consequence of PAX6 deficiency15. In agreement with previous findings, we also see abnormal or absent pineal glands in our entire patient population. This finding is consistent with sleep regulation deficits in persons with aniridia reported in other studies28. The corpus callosum has also been a structure commonly evaluated in aniridia MRI studies, with many reporting reductions in corpus callosum thickness and severe agenesis9,19,20. However, we found very few patients present with a reduced or abnormal corpus callosum, and propose that the slight reduction we see in three of our patients falls within normal population variation. Unlike most previous studies, we examined the optic chiasm, and found a reduction in the structure in more than half of our patients. This reduction could be a developmental consequence of the disorder or a progressive phenotype associated with reduced levels of PAX6. Anatomical abnormality findings seem to be highly dependent on population sample, and a larger collective sample in the literature will help us get closer to understanding common disease traits and variation.
As described above, we observed a reduction in the anterior commissure in every patient we evaluated and a reduction of the posterior commissure in five patients, but no individuals completely lacked either structure. Our study utilized a high resolution 3T MRI for data acquisition, while most other studies used a 1.5T MRI. Signal to noise ratio from 3T MRIs are almost double that of 1.5T MRIs, which will lead to an improved image quality and resolution from the 3T magnet29. We propose that the difference in observing a reduced versus absent anterior/posterior commissure may be due to a difference in scan resolution between images captured from a 3T versus 1.5T magnet. We see multiple patients in our group who have a severely reduced anterior commissure, and identifying this abnormality using a 1.5T magnet may be more difficult than when using a 3T. Additionally, the posterior commissure is smaller than the anterior commissure naturally, making it more difficult to distinguish between presence and absence in a scan. Lower scan resolution may not capture small structures such as these commissures, especially if they are reduced in size, leading to a false judgment of their absence.
A recent study has found an age component to cortical thickness in aniridia patients when compared to healthy individuals. The study found that in patients with aniridia there is an accelerated reduction in cortical thickness of the inferior parietal lobe and prefrontal/premotor areas in both brain hemispheres23. Adding to this age component seen in the Yogarajah (2016) study, there are also population differences in brain anatomy, even within healthy groups, between younger subjects and older subjects30. Additionally, as we show in our study, there are anatomical abnormalities even within healthy, unaffected participants. This makes it vitally important for careful selection of comparison subjects, and presents a caveat for interpreting differences we see in this and other clinical populations.
In addition to abnormal structural findings in patient populations with aniridia, multiple studies have assessed volumetric differences in grey and white matter in the brains of aniridia versus healthy comparisons. Similar to the findings in gross structural differences, much variation exists between reports. Some studies show an increase in grey matter volume in aniridia patients compared to healthy control groups, while others find both increases and decreases depending on brain region21,22. Changes in white matter findings follow the same suit with some reports of reductions in white matter and others finding both reductions and increases21,22. Even more interestingly, structures, such as the anterior commissure, posterior commissure, and pineal gland, show no deviation from healthy in these group-wise comparisons. This suggests that either the abnormalities seen in these structures are not as common among aniridia patients as previously thought, or that the characteristics of anatomical changes observed in aniridia patients have a high degree of variability within the population. Due to the consistency of abnormalities in structures like the anterior commissure in our study, as well as others, we predict the latter explains this discrepancy. This explanation is supported by the consistency of our results from volumetric and visual inspection of the corpus callosum in the current dataset. It is also possible that the differences observed across and within studies of these structures are a result of scan resolution and inconsistency of structure localization or plane of imaging to capture them given their small size and overall individual anatomical variation.
The current study investigated anatomical brain abnormalities correlated to aniridia in a new population sample in an effort to serve as a comparison to previous studies. Our aim was to contribute to what is known about the distribution of neuroanatomical phenotypes in the aniridia population as a whole. Although we found similar neuroanatomical abnormalities as previous studies, we find the severity is not as great as previously reported. The anterior commissure and pineal gland seem to be the structures most affected in the aniridia patients we examined, and we do see abnormalities in the posterior commissure, corpus callosum and the optic chiasm, albeit at lower frequency than previously reported. We believe the neuroanatomical abnormalities seen in aniridia populations have a high level of variability, and future studies should be aimed at collecting more patient MRI scans so that the breadth of abnormalities can be assessed.
MRI sessions were completed after written informed consent was obtained and MRI safety screening was completed. All activities were approved by the Institutional Review Board of the University of Georgia prior to subject recruitment and data collection. All individuals who participated in this study provided consent for their demographic, mutation information, and images to be published.
Dataset 1: Aniridia and healthy comparison MRI data: Structural MRI DICOM files for 12 aniridia and 12 healthy comparison individuals. Files are in DICOM format and labeled according to subject I.D. found in Table 1. These files can be opened using SPM software run in Matlab or OsiriX DICOM viewer (see Methods section). doi, 10.5256/f1000research.11063.d15379931
Mutation information that has been presented here is also available through PAX6 Allelic Variant Database (http://lsdb.hgu.mrc.ac.uk/home.php?select_db=PAX6).
AMB and JDL conceived this study as part of a large neuroimaging effort in this clinical population. JEP provided technical expertise on MRI imaging and data acquisition, and assisted AMB with experimental design and data collection. MKG analyzed the data first followed by JDW, who analyzed the data second (independently) to determine structural abnormalities in each brain. MKG and AMB prepared the first draft of the manuscript. All authors were involved in the revisions of the draft manuscript and have agreed to the final content.
This research was supported by a grant from the Sharon Stewart Aniridia Research Trust to AMB and JDL.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AMB is a Franklin Foundation Neuroimaging Fellow and an ARCS Scholar.
Collection of magnetic resonance imaging data was made possible by the University of Georgia Bioimaging Research Center. J. Branson Byers participated as an undergraduate researcher through the Center for Undergraduate Research Opportunities at the University of Georgia. The authors wish to thank Ms. Kimberly Mason for her assistance with data collection and subject screening, Ms. Jill Nerby, Director and Founder of Aniridia Foundation International, and the participants in this study for their support of this research.
Grey matter volume, white matter volume, total brain volume, and corpus callosum area of aniridia and matched subjects. Ratio refers to corpus callosum area/total brain volume. Subject ID: Numbers are matched subjects, A refers to aniridia subjects, C refers to healthy comparisons.
| ANIRIDIA | |||||
|---|---|---|---|---|---|
| Subject ID | Grey Matter Volume (mm3) | White Matter Volume (mm3) | Total Volume (mm3) | Corpus Callosum Area (mm2) | Ratio: CC area/total volume |
| 1A | 795957 | 654158 | 1450115 | 774 | 0.000534 |
| 2A | 711626 | 604800 | 1316426 | 552 | 0.000419 |
| 3A* | 683393 | 585559 | 1268952 | 712 | 0.000561 |
| 4A* | 568045 | 505360 | 1073405 | 584 | 0.000544 |
| 5A | 595209 | 573664 | 1168873 | 648 | 0.000554 |
| 6A | 543481 | 496139 | 1039620 | 542 | 0.000521 |
| 7A | 602438 | 545307 | 1147745 | 566 | 0.000493 |
| 8A | 646003 | 616039 | 1262042 | 732 | 0.00058 |
| 9A* | 621706 | 607971 | 1229677 | 684 | 0.000556 |
| 10A | 533370 | 495092 | 1028462 | 713 | 0.000693 |
| 11A | 623640 | 592662 | 1216302 | 652 | 0.000536 |
| 12A | 528675 | 489748 | 1018423 | 555 | 0.000545 |
Subject ID used in this paper, MRI DICOM ID for DICOM file matching, and Person ID from Bobilev et al. 201610.
Supplementary Figure 1: Corpus Callosum Cross-sectional area and brain volume comparisons. (A) Representation of manually traced corpus callosum in a mid-sagittal slice. (B) Corpus callosum area. (C) Grey matter volume. (D) White matter volume. (E) Total brain volume. (F) Ratio of corpus callosum area to total brain volume. Line in each graph represents average of group. No statistically significant differences were observed for any of the measurements depicted in this figure (p < 0.05).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Competing Interests: I am a co-author on several of the cited references, in particular several reporting results of similar studies (refs 17, 18, 19, 23 and some others.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 01 Sep 17 |
read | |
|
Version 1 13 Mar 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)