Keywords
scalp EEG, electroencephalogram, muscle artifact, independent component analysis, seizure
This article is included in the INCF gateway.
scalp EEG, electroencephalogram, muscle artifact, independent component analysis, seizure
We have substantially revised the manuscript in order to address the concerns of the three reviewers. In an effort to more transparently convey effect size, we have revised our statistical approach by performing the student’s paired t-test and providing the reader with the t-values. We also correct for multiple comparisons using the Holm-Bonferroni method.
In the methods section we provide greater detail regarding the AR2 methodology and also indicate that the experimental dataset was used to derive parameters which could have overestimated the efficacy of the approach. In the introduction and discussion, we offer improved explanations of the approach and results derived from an expanded body of literature.
We have slightly modified Figure 1, Figure 2, Figure 4, and Figure 5.
See the authors' detailed response to the review by David M. Groppe
See the authors' detailed response to the review by Patrícia Figueiredo and Rodolfo Abreu
The scalp electroencephalogram (EEG) is a critical diagnostic tool in the evaluation of seizures, but artifact from muscle contraction often limits its use because of the obscuring of the cerebrally generated potentials. This problem is present in 11% of ictal EEGs overall and up to 70% of frontal lobe seizures1–3. The inability, or lack of precision, to discern the seizure-onset zone from scalp EEG often necessitates additional testing, including (positron emission tomography) PET, magnetoencephalography, ictal Single-photon emission computed tomography (SPECT), and intracranial EEG4. Each of these tests adds undesired time and cost to the evaluation.
Digital filters are the common approach to maximizing the likelihood of identifying a seizure-onset zone from EEG with muscle artifact. This filtering reduces muscle artifact by attenuating all frequencies beyond a selected value5, but it may impair the integrity of the EEG recording since brain-generated potentials may be in the same frequency band6,7. Recently, new technologies to reduce muscle artifact based on independent component analysis (ICA)8–10 have become available. ICA derives spatial features that can remove artifacts that have static scalp topographies and time courses of activity that are distinct from that of EEG sources. ICA artifact correction is necessarily imperfect and will remove some neurogenic components of the EEG as well. However, the degree of EEG distortion may be negligible and ICA has proven effective at removing EMG and ocular artifacts from EEG data recorded from normal individuals in laboratory settings11–20. Prior studies have demonstrated that ICA-based methods improve the interpretation of artifact-laden ictal EEG recordings; in these studies researchers manually performed the ICA analysis prior to performing the EEG interpretation15,16. Automatic artifact reduction using ICA17–19 has become commercially available and is included in the latest versions of popular EEG viewer software20. Ictal scalp EEG recordings present extraordinary challenges to ICA artifact reduction algorithms because the number of EMG artifact sources increases.
Despite the utilization of these software products by neurologists around the globe, the clinical benefit has not been established. It is also unknown if the new approaches introduce confounding artifacts that may lead to erroneous interpretations.
The goal of this study was to assess the validity of a commercially available EEG artifact reduction tool (AR1) that uses different montages and within electrode analysis to identify artefactual independent components20, and compare its validity to a novel automatic artifact reduction tool (AR2), which was developed at the University of California Los Angeles on the basis of inter-reader agreement, confidence, and congruence with other clinical findings.
The custom software algorithm involved importing EEG scalp recordings as European Data Format (EDF) files in Matlab 8.4 (Mathworks, Natick, MA). Prior to performing ICA to remove muscle artifact, the algorithm first identified epochs of the scalp EEG record contaminated by muscle artifact and determined the electrodes that were suspected of having high recording impedance during that epoch. The purpose of these calculations was to exclude these electrodes from the ICA calculations.
The imported EEG was band pass filtered (16–70 Hz) using a 500th order finite impulse response filter, i.e. FIR1 in referential montage. We then calculated the normalized instantaneous amplitude of the band-pass filtered signal using a Hilbert transform. This signal was smoothed using moving averaging, and the algorithm identified the longest epoch in which the time series remained greater than one standard deviation. We next calculated the normalized mutual information (MI)21 adjacency matrix across all scalp electrode contacts during the (16–70 Hz) band-pass filtered artifact epoch of greatest duration and assigned each scalp EEG electrode a single MI value derived from the maximum pairwise MI values in the adjacency matrix. We then determined if this maximum mutual information value exceeded a threshold value defined by visual inspection of the scalp EEG used in the experimental dataset, and if that electrode should be included in subsequent artifact reduction processing. If the recording lacked an artifact epoch, or all channels were excluded, artifact reduction was applied to the referential recordings from all recording electrodes.
The high pass filtered (>16 Hz) scalp EEG was then separated into consecutive 120-second trials (24,000 data points) and each trial was processed using CUDAICA22,23. A 120 second trial length was chosen to optimize processing time. The purpose of the ICA was to separate the (>16 Hz) seizure activity, from the (>16 Hz) muscle artifact. The 16 Hz cut-off for the filter was chosen to isolate the vast majority of the muscle artifact. Independent components that explained an amount of variance above a particular threshold were excluded from the signal. The threshold was selected on the basis of the values of the raw and normalized mixing matrix (i.e. inverse weight matrix) calculated in each of the ICA iterations. We assumed that the last myogenic component and first neurogenic component can be differentiated on the basis of the inverse weight matrix, which provides the spatial distribution of each component, and identifying the independent component that account for the most variance with a focal spatial topography17 defined on the basis of exceeding a normalized threshold of two standard deviations in at least one electrode of the inverse weight matrix. This threshold was chosen on the basis of visual inspection of the EEG in the experimental dataset and resulting independent components.
The pruned EEG calculated for each 120 second trial of EEG (i.e iteration of CUDAICA) was concatenated, and subsequently the entire raw ictal EEG was low pass filtered (<16 Hz) using a 500th order symmetric digital FIR filter, and the resulting low pass filtered EEG was reconstituted by addition of the waveforms with the high pass (>16 Hz) filtered EEG, following the exclusion of the independent components suspected to represent muscle artifact. The reconstituted and modified ictal EEG was exported from Matlab format to EDF for subsequent visual analysis.
All computations were carried out using compiled Matlab 8.4 custom scripts on a cluster of HP SL230s Gen 8 ES-2670 nodes with dual-eight-core 2.6 GHz Intel ES-2670 central processing units, 4 GB of memory per core, and NVIDIA Tesla graphics processing units. Minimal system requirements for operating AR2 include Matlab v8.4 or above, an Intel Xeon CPU, 2 GB of memory, a NVIDIA GPU, which is CUDA compatible, and CUDAICA. For scalp EEG files exported from Neuroworkbench (Nihon-Kohden, Irvine, CA, USA), executing the AR2 software method requires only inputting the file name of the EDF file of interest at the command line. For EDF files exported from other equipment manufacturers, full automation of the AR2 software method can be easily accomplished with simple modifications of the input parameters.
We tested AR2 retrospectively using 23 seizures from eight adult patients with suspected focal-onset seizures undergoing evaluation at the UCLA Seizure Disorder Center. The patients and seizures were selected by S.A.W, whom was not a reviewer, from a review of consecutive clinical neurophysiology case conference presentations between January 1, 2014 and December 1, 2015 and based on case conference consensus that the ictal EEG records were uninterpretable due to muscle artifact contamination when reviewed with conventional digital filtering. For each of these patients, between 1–4 uninterpretable seizures were selected for inclusion in the study on the basis of a lack of ECG, electrode, and salt bridge artifact by S.A.W. Clinical data for each patient included seizure semiology, inter-ictal epileptiform abnormality, unobscured findings and radiological reports from MRI, PET, SPECT scans. The EEG and clinical records were deidentified and research informed consent was not required. This study was approved under UCLA IRB#15-001481. The video EEGs were acquired using a EEG-1200 amplifier (Nihon-Kohden, Irvine, CA) at a sampling rate of 200 Hz, low frequency cut-off 0.08 Hz. Electrodes were placed according to the 10–20 international system with the additional anterotemporal electrodes at T1/T2. The duration of the exported EEG recording included the entire seizure and a 2–3 minute peri-ictal epoch.
AR1 was the commercially available Persyst v12 artifact reduction software20 (Persyst Development, San Diego, CA). The methods are proprietary. AR2 was developed by S.A.W and involved a two-step procedure consisting of a custom algorithm. EEG processed by AR2 was also interpreted using the Persyst v12 artifact reduction software.
The AR1 and AR2 processed data were reviewed in Persyst v12 without video by 26 neurologists with a specialization in EEG, 20 of whom were board certified. The readers were blinded to which records received AR1 or AR2, and each reader reviewed the 46 seizures in random. Following review of each ictal record, the reader completed a multiple choice questionnaire (Supplementary File 1), which assessed ability to visualize seizure-onset (Y,N) lateralize seizure-onset (L,R,N), locate the region of ictal onset (anterior temporal, anterior frontal, mid-temporal, temporal-parietal-occipital, occipital, none), and self-identify confidence of interpretation on a 5 point scale [(5) entirely confident (4) somewhat sure (3) probable (2) not confident (1) unlikely i.e. slight probability] for each measure. When time of onset, laterality, or the seizure onset region was not assigned the confidence was taken as (0). Readers were not provided with a definition of seizure-onset.
During the interpretation of the ictal EEG processed by AR1 or AR2, no restrictions were placed on the use of Persyst v12 built in EEG filters (low-pass, high-pass, band-pass), or changes to montage. A comment in each recording was used to demarcate the time prior to the clinical seizure but not the EEG onset. The assessment was not time limited.
Differences in EEG interpretation utilizing AR1 and AR2 were assessed using the paired student’s t-test and the McNemar test on paired nominal data. The Bonferroni-Holm method was used to correct for multiple comparisons. Agreement across readers (Y,N,L,R), using either AR1 or AR2, was calculated using the inter-class correlation coefficient (ICC). For these outcomes, missing values were imputed to be in between non-missing values, and were analyzed using cumulative logit mixed effects models, which capture this ordering in the values and accounts for the clustering of readings into patients, and seizures within patients. Agreement across readers for onset region was calculated using a Fleiss kappa and treating the missing values as a category of response. Errors are given as standard error of the mean (s.e.m), unless otherwise specified.
We applied the AR2 method developed at UCLA to the 23 seizures in the dataset. The method was automatic and unsupervised and separated the high-pass filtered (> 16 Hz) scalp EEG recordings into putative neurogenic and myogenic components (Figure 1). After pruning the putative myogenic components, the putative neurogenic components were reconstituted with the low-pass filtered (< 16 Hz) scalp EEG (Figure 2). The AR2 and AR1 processed scalp EEG recordings were subsequently inspected by the 26 specialists (Figure 3).
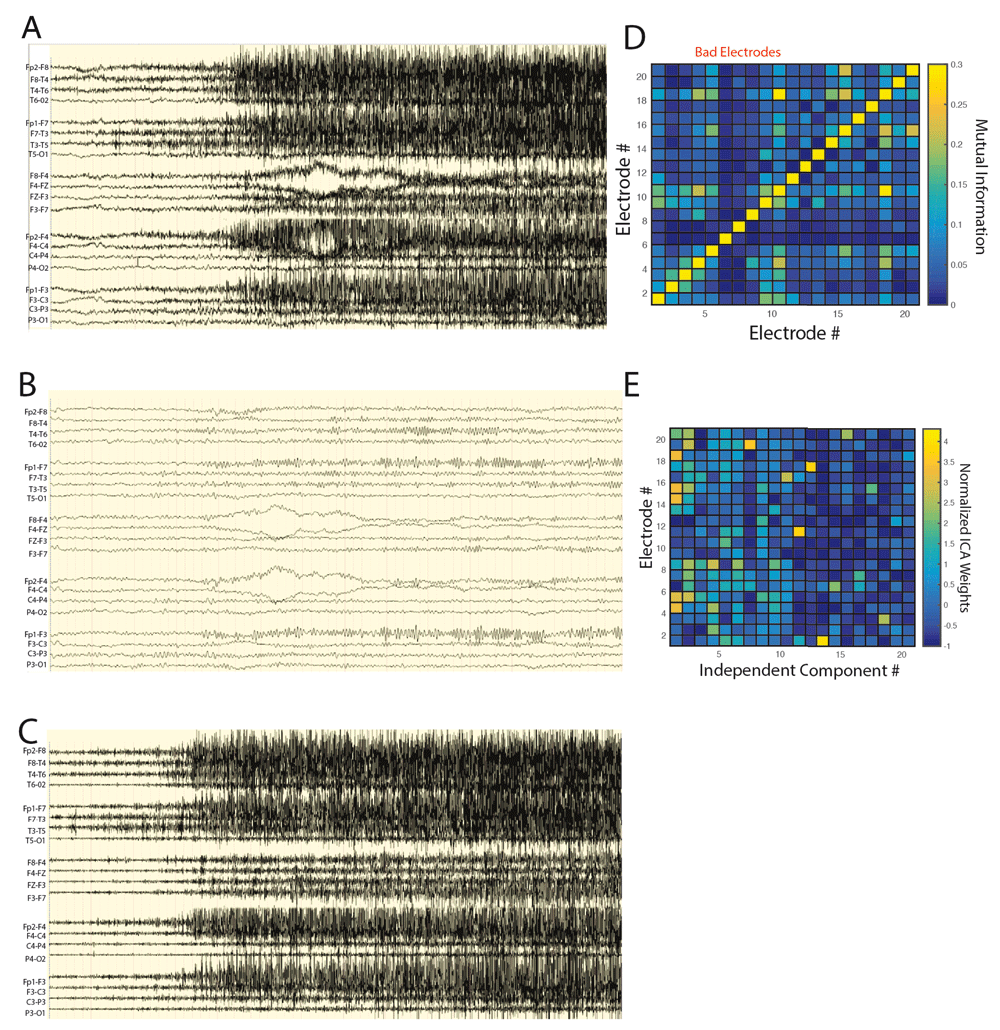
The AR2 method automatically separates independent components containing myogenic from neurogenic potentials in the beta and gamma band on the basis of spatial topography and explained variance. A. Unprocessed scalp ictal EEG recording that was deemed uninterpretable. B. The same epoch after applying a low pass (<16 Hz) filter demonstrating a lack of a convincing ictal rhythm. C. The ictal epoch after applying a high pass (> 16 Hz) filter demonstrating dense muscle artifact. D. An example of a mutual information adjacency matrix calculated during an epoch of artifact in the high pass (> 16 Hz) filtered scalp EEG recording. Three scalp electrode recordings exhibited relatively low mutual information with all other electrodes and were designated poor quality and excluded from further processing to optimize INFO-MAX ICA based artifact reduction. E. The normalized inverse weight matrix of all independent components across scalp electrode recordings for the seizure in panel A. Independent components 1-13 exhibited strong focality and were designated as containing myogenic potentials, while independent components 14 and above were designated neurogenic.

Reconstitution of the low pass (<16 Hz) ictal scalp EEG with the high pass (>16 Hz) neurogenic independent components reveals a clear ictal onset. A. The tentative neurogenic independent components (A1) and myogenic independent components (A2) derived from INFOMAX ICA processing of the high pass (> 16 Hz) filtered ictal scalp EEG recording are shown. The largest amplitude activity in the neurogenic components are evident frontally and in the left hemisphere. B. The low pass filtered ictal scalp EEG suggests a possible left frontal onset but a convincing ictal rhythm is lacking. C. Reconstitution of the low pass EEG with the neurogenic high pass (> 16 Hz) independent components results in an ictal EEG that demonstrates a more convincing left frontal onset consisting of beta-gamma oscillations with some clear phase reversals in F3 and F7.
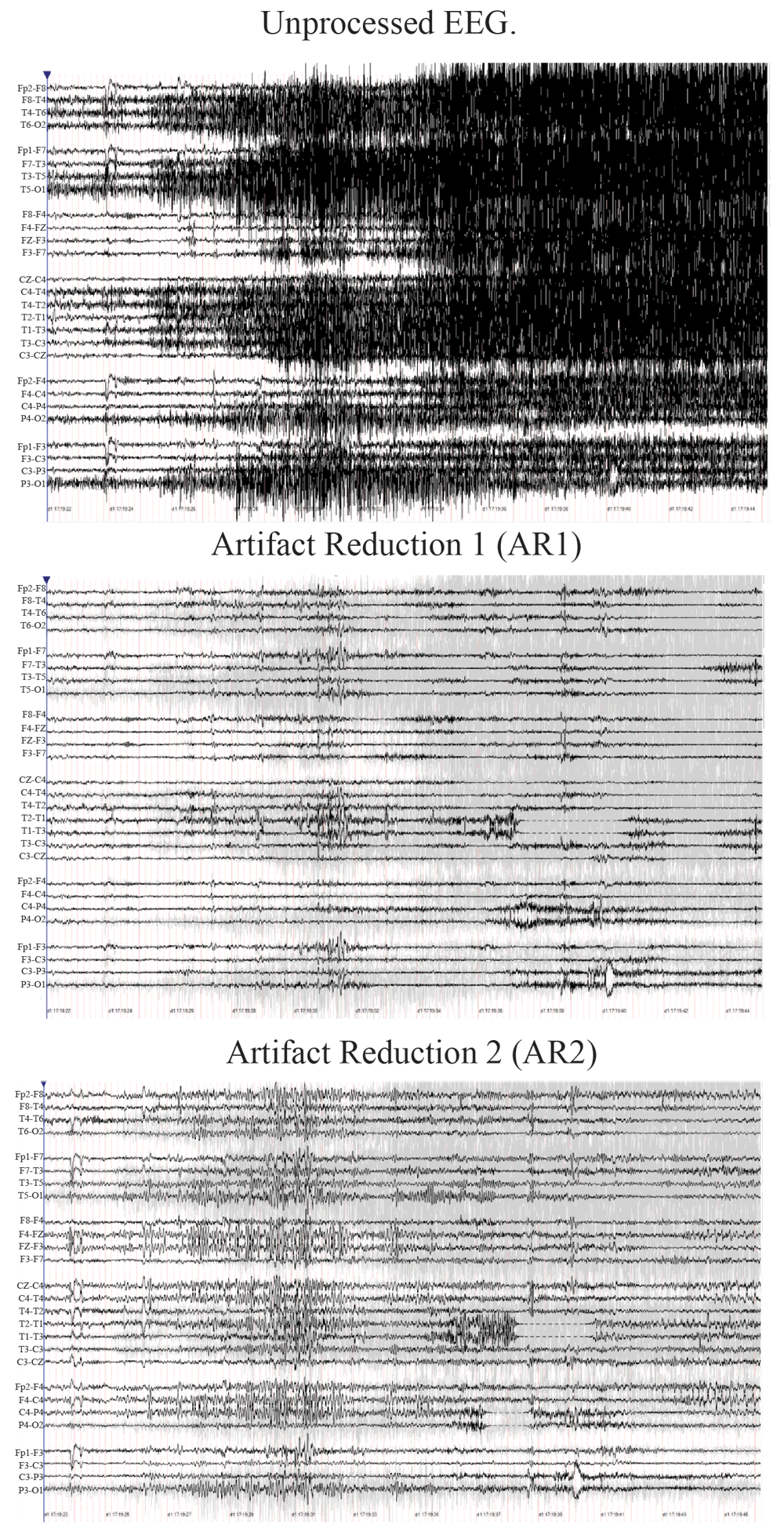
Ictal scalp EEG recording from seizure 18 prior to artifact reduction processing (top), after processing with artifact reduction methodology 1 (AR1, middle), and after processing with artifact reduction methology 2 (AR2, bottom). Only processing with AR2 reveals a right hemispheric onset followed by clear spread to right frontal regions.
Across the 23 seizures considered previously uninterpretable with digital filtering (Table 1) two-thirds of the readers were able to delineate the time of seizure-onset for 10 of the 23 using AR1, and 15 of the 23 using AR2 (Figure 4A, n=23, paired t-test p<0.01, t=3.83). Across the 23 seizures, the mean confidence measure for the determination of seizure-onset was 2.68 +/- 0.19 (probable-not confident) when AR2 was utilized and 2.19 +/- 0.18 (not confident) with AR1 (Figure 5A, d.f.=22, paired t-test, p<0.01, t=4.33). The inter-class coefficient (ICC) was 0.26 (95% Confidence Interval (CI) 0.21-0.30) with AR2, and 0.15 (95% CI 0.11-0.18) with AR1 (cumulative logit mixed effects models, p=0.333).
Clinical description of patients and ictal EEG laterality and focus assignments with AR1 and AR2. Abbreviations (L:left, R:right), PET findings refer to hypometabolism, SPECT findings to hyperperfusion. The focus was determined on a majority basis across all the assignments made by the readers for a subject’s seizure(s).
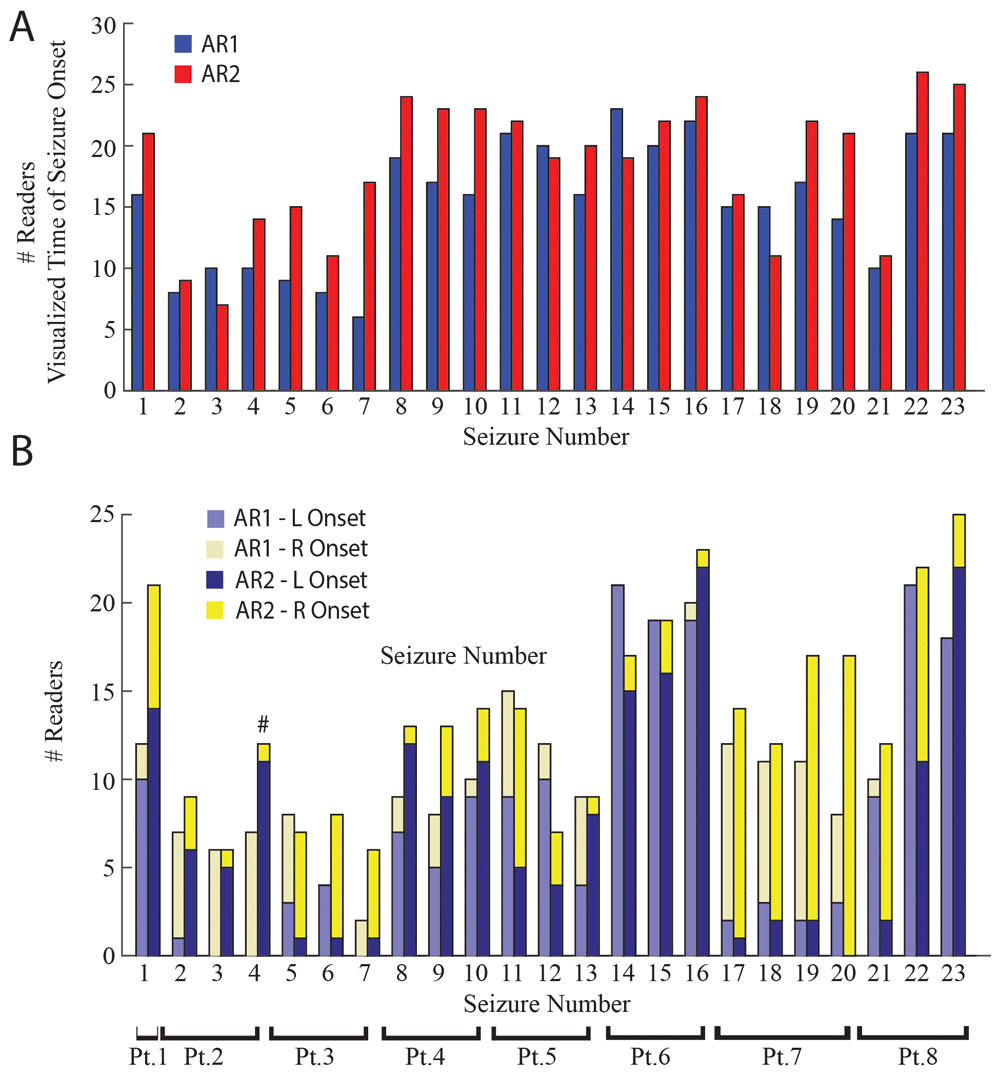
More readers could visualize the time of seizure onset, and assign laterality to seizure onset utilizing AR2 as compared to AR1, and the assigned laterality of seizure onset sometimes differed between the two methods. A. Bar plot of the number of readers whom visualized the time of onset for each seizure utilizing AR1 (blue) or AR2 (red). Across seizures more readers visualized seizure onset utilizing AR2 compared with AR1 (p<0.01). Asterisks indicate statistically significant differences between the two methods in individual seizures (McNemar, p<0.05, Bonferroni-Holm corrected). B. Stacked bar plot of the number of readers selecting a left- or right-sided seizure onset utilizing AR1 (light blue, left; light yellow, right) or AR2 (dark blue, left; yellow, right). Across seizures more readers lateralized seizure onset utilizing AR2 compared with AR1 (p<0.01). Asterisks indicate statistically significant differences in individual seizures (McNemar, p<0.05, Bonferroni-Holm corrected), number sign indicates a significant change in the determination of laterality utilizing AR2 compared to AR1 (McNemar, p<0.05, Bonferroni-Holm corrected).
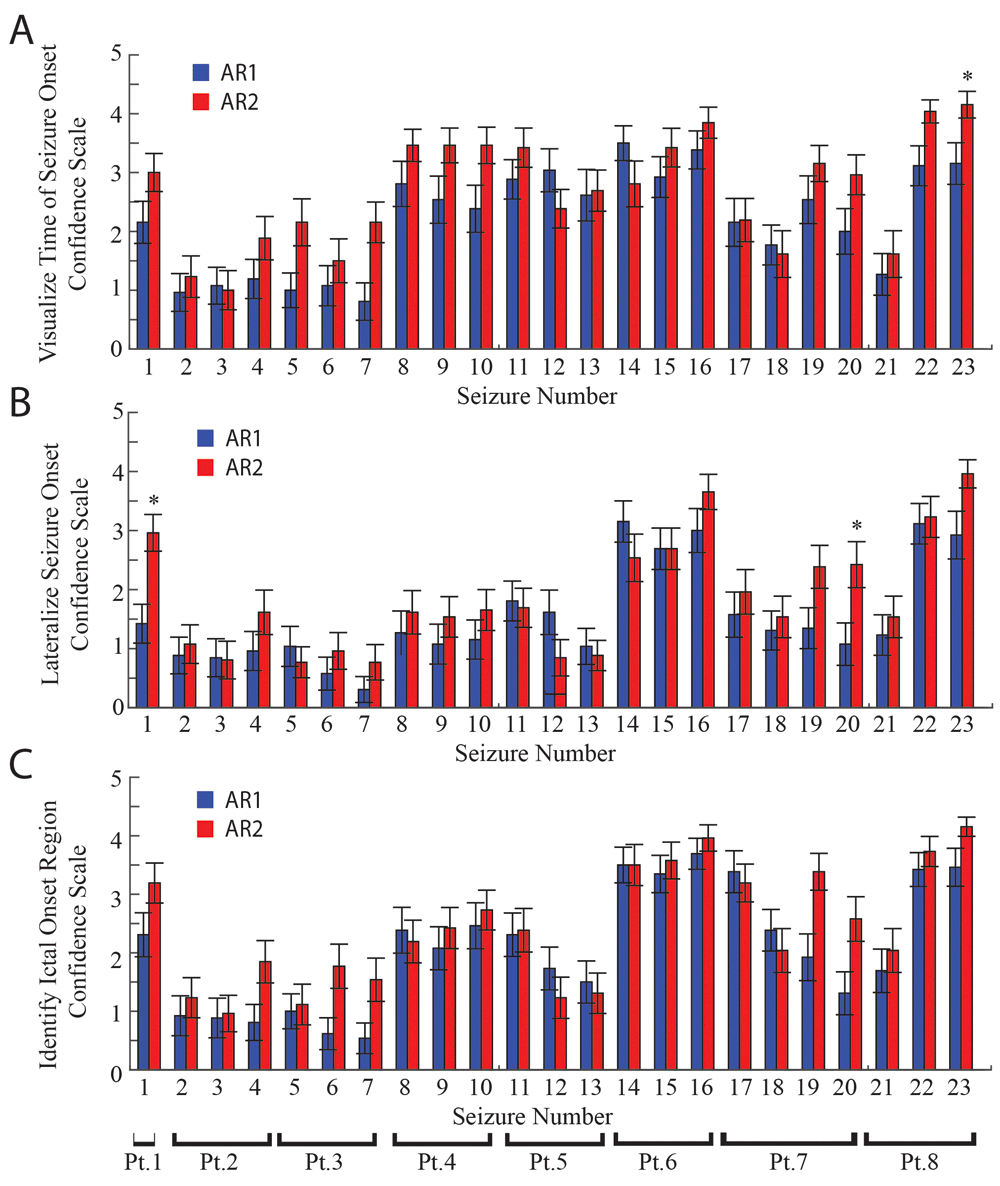
A. Bar plot of the mean confidence scale values for visualizing the time of seizure onset for the 23 seizures interpreted utilizing AR1 (blue), and AR2 (red). Across seizures, confidence scale values were greater when AR2 was utilized as compared with AR1 (p<0.01). Asterisks indicate differences in confidence values in individual seizures (p<0.05, Bonferroni-Holm corrected). Error bars are calculated as s.e.m. B. The respective mean confidence scale values for seizure onset lateralization. C. The respective mean confidence scale values for seizure focus localization. Across seizures, confidence scale values for lateralizing seizure onset, and identifying the seizure focus were greater when AR2 was utilized as compared with AR1 (p<0.05).
Compared with identifying the time of seizure-onset, fewer readers could lateralize seizure-onset after either AR1 or AR2 (Figure 4B, d.f.=22, paired t-test, p<0.01, t=8.08 AR1, t=8.56 AR2). However, more readers were able to lateralize seizure-onset using AR2 compared to AR1 (Figure 4B, d.f.=22, paired t-test, p<0.01, t=3.30) and readers were more confident with AR2, although both methods did not produce high levels of confidence. The mean confidence measure for seizure-onset lateralization was 1.87+/- 0.198 (not confident-unlikely) for AR2 and 1.54+/- 0.176 (not confident-unlikely) for AR1 (Figure 5B, d.f.=22, paired t-test, p<0.01, t=2.85). The ICC was equivalent (cumulative logit mixed effects models, p=0.501) for AR1 (ICC=0.33 95% CI 0.30-0.37) and AR2 (ICC=0.28 95% CI 0.25-0.31). For localizing the region of seizure-onset reader confidence (Figure 5C), and agreement was very low (Figure 6, AR1 Fleiss’ kappa = 0.1199, 95% CI = 0.116-0.124, AR2 Fleiss’ kappa = 0.121, 95% CI =0.118-0.125). For one of the seizures, the laterality assignments were different when AR2 was used as compared to AR1 (Figure 4B, McNemar p<0.05).
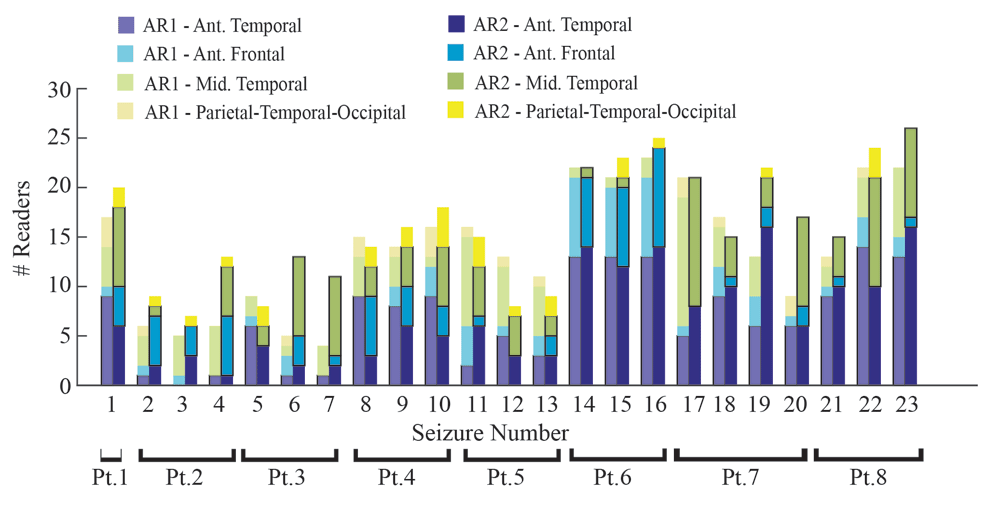
Stacked bar plot of the ictal onset region assignments using either AR1 (lighter colors) or AR2 (darker colors) for all 23 seizures. Overall, across seizures, more readers were able to render an assignment using AR2 as compared to AR1 (p<0.05). Inter-reader agreement using for assigning the ictal onset region was marginal using either AR1 or AR2.
We identified the patients with at least two consistent clinical findings that lateralized the suspected seizure-onset zone (SOZ). Compared to AR1, more readers were able to render seizure-onset laterality assignments using AR2, and these assignments were more often congruent with other clinical data (Table 2). These clinical findings included seizure semiology, onset of seizures without EEG obscuration, structural MRI, PET, or SPECT findings. If any of the clinical findings were contradictory with respects to the laterality of the suspected SOZ, the SOZ was designated unknown. Overall, 4 patients (#1,4,5,6) had clinical findings that supported a left-hemispheric SOZ, and 1 patient (#7) had clinical findings that supported a right-hemispheric SOZ (Table S1). Among the 5 patients with clinical seizure onset lateralization based on independent data, if the reader lateralized the seizure-onset to the left using AR2 they were correct in 95.9% (95% CI 85.7-98.9%) of cases, but using AR1 they were correct in 91.9% (95% CI 77.0-97.5%) of cases (Table 3, p<0.0607).
Contingency table of the agreement between seizure-onset laterality using AR1 (left), and AR2 (right) and the laterality of seizure-onset assigned on the basis of other clinical data for all the study patients and seizures. Note that clinical seizure-onset lateralization was not available for all patients, and when readers rendered a laterality decision that matched the laterality based on other clinical data, the assignments “agreed”.
Agreement between seizure-onset laterality assignments using either AR1 or AR2 and the suspected laterality of the SOZ assigned on the basis of other clinical data. Parentheses indicate the 95% confidence interval. “n” refers to the number of subjects.
In this study, we present a new artifact reduction software, AR2, and its application compared with a commercially available tool, AR1. 26 neurologists used the two methods to interpret 23 ictal EEG recordings that were uninterpretable due to muscle artifact when reviewed with conventional filtering. The major findings from this study include: 1) the utilization of artifact reduction software results in non-uniform interpretation of ictal EEG, with many readers not able to render assignments; 2) when readers did render seizure-onset laterality assignments it often agreed with other clinical findings; 3) although the study size was small, the AR2 software method increased the number of readers that rendered assignments, and reader confidence suggesting it aids in diagnosis.
Both AR1 and AR2 are digital signal processing software tools8,15,20 that may confound accurate ictal EEG interpretation by altering the appearance of the EEG. Digital filtering also can mislead5. One concern about AR1 and AR2 relates to the uncertainty that myogenic activity was fully removed, and neurogenic components were unaffected during waveform alteration. Specifically, the readers were not confident in their interpretations, and the determination of seizure lateralization sometimes differed between the AR1 or AR2 methods. As such, the artifact reduction methods may introduce false positive findings. This demonstrates the limits of EEG artifact reduction approaches and puts the advantages into perspective.
The reliability of localization by ictal scalp EEG in the absence of artifact is between 65–75% for lateralization24. Neurologists disagree more on the interpretation of ictal EEG processed with artifact reduction software, however the seizure-onset laterality assignments rendered by a quorum are often correct. Further refinement of this technology may successfully improve the efficiency of video-EEG monitoring and the utilization of epilepsy surgery; however, correlation with epilepsy resective surgery outcomes will be required for further validation.
With regard to AR2, the novel software method developed for this study, the slight improvement seen in ictal EEG interpretability after applying the method suggests that the algorithm can (1) sometimes produce signals that are, exclusively or mainly, EEG or EMG, and (2) identify which signals are of brain origin and which are contaminant. The effectiveness of AR2 could possibly be improved by utilizing autocorrelations to identify the myogenic independent components17
One explanation for AR2’s ability to isolate myogenic from neurogenic activity may be related to the respective dipole generators of each. ICA produces independent components that may resemble single equivalent dipoles14. Presumably, networks of myocytes exhibit shorter distance connectivity then networks of neurons that produce beta and gamma oscillations, and thus the two generators can be distinguished on the basis of the focality17 of the independent components topography.
All software code for the new AR2 software developed by S.A.W. is openly and permanently available at https://github.com/shennanw/AR2.
Archived source code as at time of publication: doi, 10.5281/zenodo.22989321
License: GNU Public License 3.
The raw scalp ictal EEG files that were analyzed in this study using AR2, as well as the scalp ictal EEG files following processing using AR2 are available from Zenodo25: Dataset 1. Validity of two automatic artifact reduction software methods in ictal EEG interpretation. Doi, 10.5281/zenodo.22109522 (https://www.zenodo.org/record/221095#.WF63m7YrLdR)
The raw data used for the comparative assessments are available from Zenodo26: Dataset 2. Validity of two automatic artifact reduction software methods in ictal EEG interpretation. Doi. 10.5281/zenodo.223329 (https://zenodo.org/record/223329#.WHN-HLYrLdQ)
S.A.W designed the study, analyzed the data, drafted and revised the manuscript, A.A.P analyzed the data, and revised the manuscript, S.V analyzed the data, and revised the manuscript, S.M revised the manuscript, D.H.W revised the manuscript, I.O analyzed the data, and revised the manuscript, M.G analyzed the data, and revised the manuscript, L.S analyzed the data, and revised the manuscript, J.L analyzed the data, and revised the manuscript, C.K.C analyzed the data, and revised the manuscript, E.C analyzed the data, and revised the manuscript, R.R analyzed the data, and revised the manuscript, I.K analyzed the data, and revised the manuscript, P.C analyzed the data, and revised the manuscript, C.B.B analyzed the data, and revised the manuscript, A.L.N analyzed the data, and revised the manuscript, M.G.H analyzed the data, and revised the manuscript, L.R analyzed the data, and revised the manuscript, A.B analyzed the data, and revised the manuscript, J.S analyzed the data, and revised the manuscript, M.A analyzed the data, and revised the manuscript, T.A analyzed the data, and revised the manuscript, A.F analyzed the data, and revised the manuscript, M.N analyzed the data, and revised the manuscript, C.S analyzed the data, and revised the manuscript, S.M analyzed the data, and revised the manuscript, D.S.E analyzed the data, and revised the manuscript’, G.W.M analyzed the data, and revised the manuscript, M.R.N analyzed the data, and revised the manuscript, M.S analyzed the data, and revised the manuscript, J.E analyzed the data, and revised the manuscript, J.S designed the study, analyzed the data, revised the manuscript.
Dr. Weiss was supported by an Epilepsy Foundation Award Research and Training Fellowship for Clinicians.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank Mrs. Sandra Dewar for her administrative assistance, and Mr. Kirk Shattuck for his technical support.
Supplementary File 1: Multiple choice questionnaire, completed by the reader after review of the ictal record.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
References
1. Urigüen JA, Garcia-Zapirain B: EEG artifact removal-state-of-the-art and guidelines.J Neural Eng. 2015; 12 (3): 031001 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroimaging, Signal processing
Competing Interests: No competing interests were disclosed.
References
1. Jung TP, Makeig S, Humphries C, Lee TW, et al.: Removing electroencephalographic artifacts by blind source separation.Psychophysiology. 2000; 37 (2): 163-78 PubMed AbstractCompeting Interests: No competing interests were disclosed.
References
1. Chaumon M, Bishop DV, Busch NA: A practical guide to the selection of independent components of the electroencephalogram for artifact correction.J Neurosci Methods. 2015; 250: 47-63 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 04 Apr 17 |
read | read |
|
Version 1 10 Jan 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)