Keywords
wash-in, low-flow anaesthesia, MAC, End-tidal concentration, sevoflurane
wash-in, low-flow anaesthesia, MAC, End-tidal concentration, sevoflurane
We are grateful to the referees for important comments on our paper. There are indeed both unexpected findings and indeed major limitations with our study. We still believe the information is of some importance. We have in the revised version addressed limitations accordingly, gas monitoring, study set up, thus no uptake or elimination of anaesthetic to blood compartment. We have also addressed potential theoretic explanations to the difference in circle system wash-in, differences in the vaporasation technology.
See the authors' detailed response to the review by Göran Hedenstierna
A rapid change in inspired anaesthetic agent is a requisite for the control of the depth of anaesthesia. Low flow anaesthesia has been increasingly adopted, as it is associated with several benefits, including conserving humidity and temperature, which improves the quality of anaesthesiai. Reducing the amount of anaesthetic agent consumed is of interest not only for reducing cost, but also for reducing environmental burden. The merits of reducing flow must not overrule safety, by maintaining adequate oxygen content in the circle, and avoiding hypoxic gas mixture, inadequate anaesthesia control and too light anaesthesia with risk for awarenessii,iii. Increasing and decreasing the circle system anaesthetic concentration during low and minimal flow anaesthesia calls for knowledge around the technique and kinetics of the anaesthetic gas used. We studied wash-in, increase and decrease of the end-tidal gas concentration, within the circle system with two anaesthetic machines, GE Aisys and Maquet FLOW-i, at different fixed fresh gas flow and fixed vaporizer settings in a test model. Our hypothesis was that the wash-in and wash-out should be fresh gas flow dependent, and that the time for reaching target end tidal concentrations would be faster for the FLOW-i device, which does not have a classical reservoir.
The two anaesthetic workstations, Aisys (GE Healthcare, Madison, WI, USA) and FLOW-i (Maquet, Solna, Sweden), including a standard CO2 absorber and a standard circle system (patient circuit, adult, disposable 1.8 m; GE Healthcare) and a Humid-Vent Filter (Teleflex, Wayne, PA, USA), were connected to a 2 L elastic test reservoir (Intersugical Ltd., East Syracuse, NY, USA).
Wash-in: time to reach a stable circle concentration; end-tidal concentration of 1 and 1.5 MAC age adjusted (40 year old male) sevoflurane (2.1 and 3.1%) was studied with the circle connected to the test reservoir. Ventilation was set at tidal volume 500 ml, respiratory rate 10 and PEEP 5 cmH2O, for both devices. The oxygen fraction was set at 0.4. Ventilation settings and FiO2 was kept constant during thire test, wash-in and wash-out- Fresh gas flow was fixed at 300, 500, 1000, 2000 and 4000 ml/minute. The vaporizer setting was fixed at 8% for both devices during the wash-in and 0 at wash-out.
The time to reach 1 MAC and the time to increase from 1-1.5 MAC circle concentration was recorded for each value, based on mean of 3 repeated tests.
Wash-out: time to decrease from 1.5 MAC to 0 gas concentration with a fixed fresh gas setting of 2500, 5000, 7500 and 10000 ml/minute.
Workstations at the department of anaesthesia at Danderyds University hospital was used for the study. Gas monitoring was done with each workstations side-stream multi-gas infra-red monitor. These analysers are 0-calibrated automatically at start of the machine. The workstations are further controlled and calibrated in accordance to the service routine of the department.
All data are presented as mean and standard deviation based on 3 repeats. The effects on time events (wash-in: increase from 0 – 1 MAC and further increase up to 1.5 MAC; wash-out: decrease from 1.5 MAC to 0 gas) between fresh gas flows and anaesthetic work-stations were calculated by ANOVA. P<0.05 was considered to be statistically significant. Data was analysed with StatView (v1.04) for MAC.
Fixed fresh gas flows had a significant impact on the speed of wash-in - time to achieve a stable circle end-tidal sevoflurane concentration of 1 MAC age adjusted (2.1%). The mean time of both machines decreased from 547 ± 83 seconds, at a fixed fresh gas flow of 300 ml/min and fixed vaporizer setting of 8%, to 38 ± 6 seconds at a fresh gas flow of 4000 ml/min. The time to further increase the circle system end-tidal sevoflurane from 1 to 1.5 MAC also showed a significant dependency on fresh gas flow: 330 ± 24 seconds, at a fixed fresh gas flow of 300 ml/min and fixed vaporizer setting of 8%, to 17 ± 11 seconds at a fresh gas flow of 4000 ml/min.
Both anaesthetic work-stations showed the same fresh-gas flow dependent for wash-in and wash-out pattern, but the Aisys showed overall a slightly faster wash-in time (Table 1 and Figure 1).
Vaporizer set at 8%, tidal volume 500 ml, respiratory rate 10, PEEP 5 and volume controlled ventilation.
| L/min | ||||||
|---|---|---|---|---|---|---|
| MAC | 0.3 | 0.5 | 1 | 2 | 4 | |
| FLOW-i | 0–1 | 618 | 342 | 141 | 75 | 42 |
| 1–1.5 | 317 | 191 | 88 | 46 | 25 | |
| Asysis | 0–1 | 477 | 232 | 99 | 48 | 33 |
| 1–1.5 | 344 | 165 | 96 | 22 | 10 | |
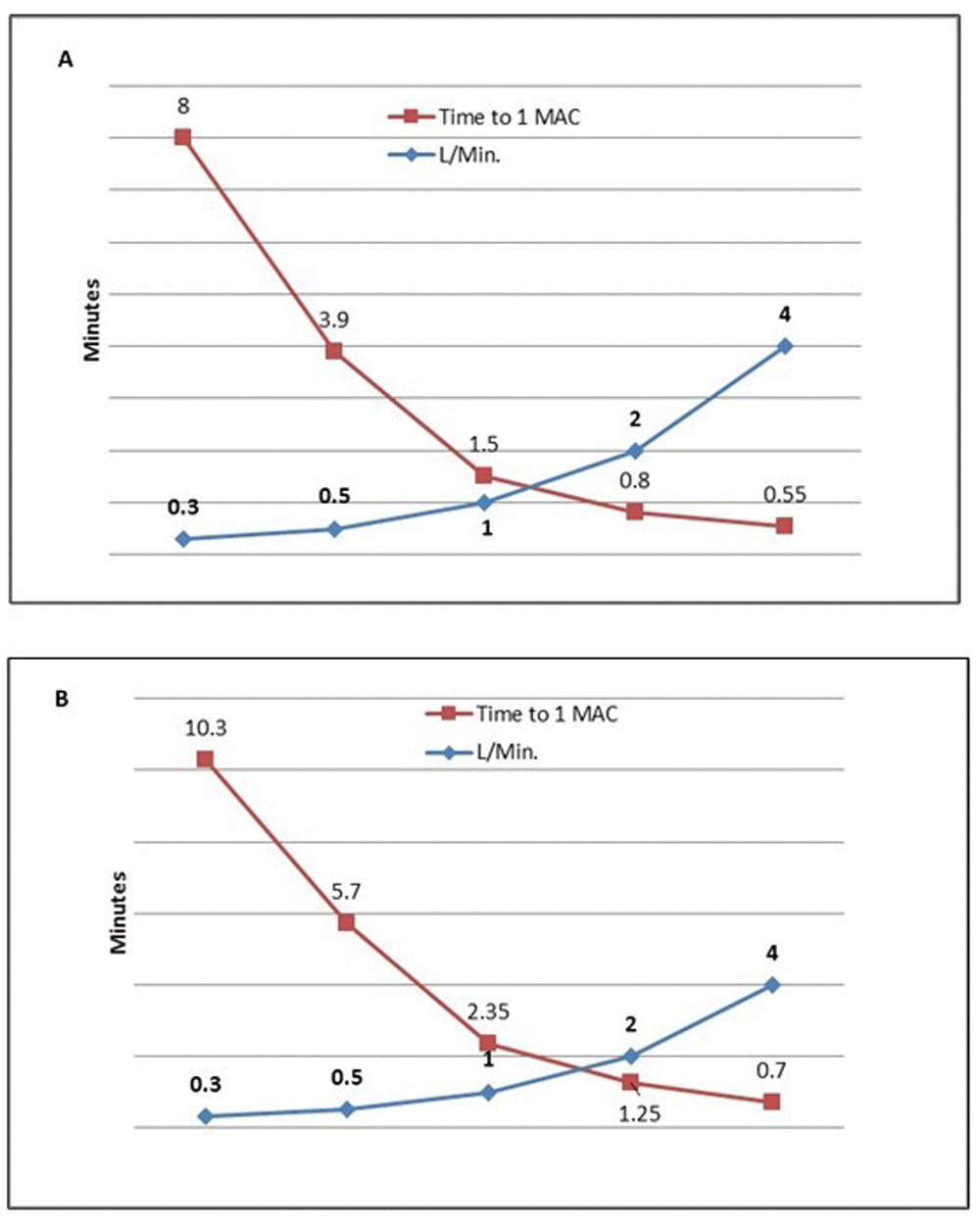
Wash-in time (minutes) to increase to 1 MAC (sevoflurane 2.1%) for (A) Aysis and (B) FLOW-i anaesthetic work stations.
Wash-in (time to reach a stable 1 MAC circle sevoflurane concentration) was achieved within 1.5 minute at a fixed fresh gas flow of 2000 ml/min for both machines tested, 48 ± 2 and 75 ± 2 seconds for the Aisys and Flow-I, respectively (p<0.001), and within 1 minute, mean 33 ± 3 and 42 ± 3 seconds, for Aisys and Flow-i at 4000 ml/min, respectively (p<0.05). A further increase from 1 to 1.5 MAC was achieved within 1 minute for both machines (22 ± 3 and 46 ± 3 seconds for Asysis and Flow-i, respectively) at a fixed fresh gas flow of 2000 ml/min. When a 4000 ml/min was used, the monitoring system was not fast enough to catch the increase for the Aisys, but recorded the increases as 25 ± 10 seconds for Flow-i.
Wash-out was likewise flow dependent, and plateaued at 7.5 L/min (Table 2 and Figure 2).
Tidal volume 500 ml, respiratory rate 10, PEEP 5 and volume controlled ventilation.
| L/min | ||||
|---|---|---|---|---|
| 2.5 | 5 | 7.5 | 10 | |
| Aysis | 15.41 | 4.25 | 2.14 | 2.15 |
| FLOW-i | 10.73 | 4.45 | 2.49 | 2.48 |
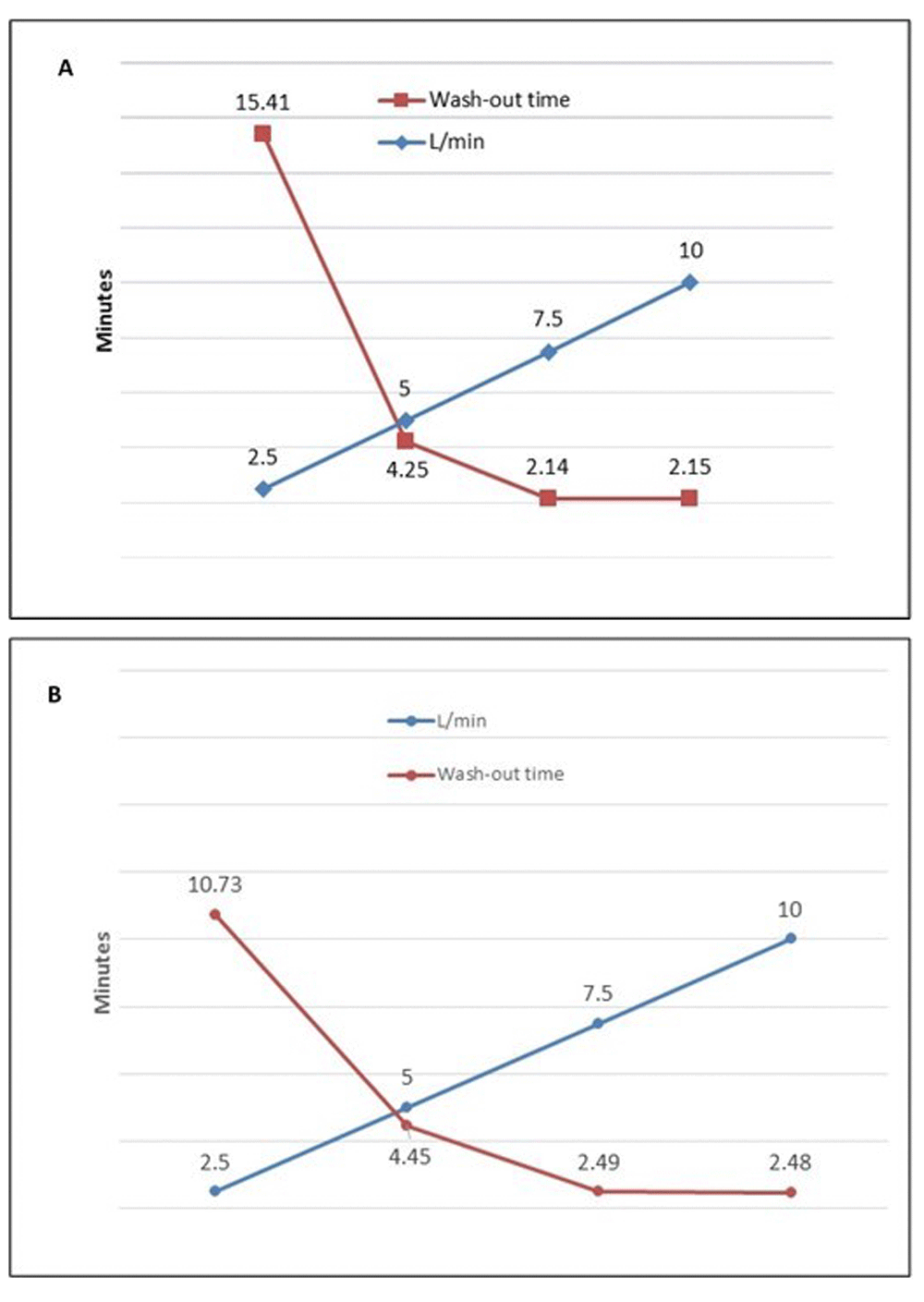
Wash-out time (minutes) for decrease from 1.5 to 0 MAC with sevoflurane at 3.1-0% in (A) Aysis and (B) FLOW-i anaesthetic work stations.
The present study was set-up to evaluate the impact of fresh gas flow on saturation of and wash-out from the circle system/test-lung set up and whether the modern anaesthesia machines performed differently. We found a clear fresh-gas flow dependency for the time to saturate and wash-out of the circle and test-lung system, as expected. Wash-in to 1 MAC and further increasing the circle concentration to 1.5 MAC decreased with the fresh gas flow, and a 1 and further increase to 1.5 MAC was achieved within 1 minute at a fresh gas flow of 4 L/min. The wash-out was not further improved between 7.5 and 10 L/min fresh gas flow.
We found somewhat surprisingly that the Aisys was slightly faster than the FLOW-i, although the FLOW-i should have a small internal gas reservoir. Lucangelo et al. studied the FLOW-i performance regarding tidal volume in case of minor leakageiv. They found the system to be highly accurate. They also described the gas flow control in detail addressing the technical features of the flow regulators. Thus, our hypothesis was a faster saturation of the circle gas with the FLOW-i technology. We cannot give any explicit reason why the wash-in unexpectedly was slower for the FLOW-i. One contributing mechanism may be differences in the vaporizer technology. According to the Maquet user's manual, during controlled ventilation “a larger proportion of the fresh gas is added during the inspiration phase, also contributing to minimizing agent consumption”. The Flow-i vaporiser only injects anaesthetic agent during inspiration and is inactive during the expiratory phase of the cycle which may contribute to the difference in wash-in. The Aisys delivers vapour continuously, like a conventional vaporizer, which might explain why wash-in to a test lung is faster. One should also acknowledge that the gas measurements were done by the built in multi-gas analysers. To what extent that could have impacted the results cannot be stated.
Dosch et al.v studied the change in circle gas composition in three anaesthetic machines and found that fresh gas flow and breathing system volume have the biggest effect on time to equilibrium. In a previous study, we analysed the wash-in of desflurane and sevoflurane during fixed fresh gas flow and vaporizer setting with the Aisys anaesthesia workstationvi. We found, as expected, desflurane to be associated to a significantly faster wash-in compared to sevoflurane with a significant impact from the fresh gas flow: The increase from 0.5 L/min to 1.0 L/min in fresh gas flow reduced the time to reach 1 MAC age adjusted end-tidal concentration from 15.2±2.4 minutes to 6.2±1.3 minutes. We found in that study a rather large variability for sevoflurane, which we considered was related to a combination of circle system gas saturation and uptake.
Kern et al. studied the saturation of neonatal anaesthesia systemsvii. They found huge differences in the time to reach the end tidal concentration above 95% of inspired. They also found wash-in times to decrease with higher fresh gas flows and higher minute ventilation rates; however, they saw that the effect of doubling fresh gas flow was variable and less than expected. Struys et al. made a study much like ours comparing the Zeus apparatus with direct injection of inhaled anaesthetics and the Primus apparatus using a classical out-of-circle vaporizerviii. They found the Zeus to have a faster time course, but their study set-up was different from ours; they used fresh gas and auto control modes, providing a high initial fresh gas bolus. We compared the novel FLOW-i with a similar injection technique and without classical reservoir, and the Aysis with a more classic design. Carette et al. studied the performance of the automatic control mode of the FLOW-iix. The possibility to use an automatic algorithm to reach desired circle, end-tidal concentration is an interesting option and we plan to do further studies assessing the automatic technique. One limitation of the study was that it is an entirely experimental study.
There are several limitations with our study. We studied only wash-in and wash-out in a test lung and indeed there was no uptake of anaesthetic vapour from the breathing circuit and likewise no anaesthetic agent elimination from the blood during wash-out. We used the built in gas monitors. It would have been of value to have had a free-standing well calibrated multi gas sensor. One may also argue that statistical differences seen may not translate into clinical important differences. Further studies assessing the benefits and limitations with the new and costly anaesthetic works stations are warranted.
In conclusion, wash-in, saturation of and wash-out of the circle system is fresh gas flow dependent. A 1 MAC can be reached within 1 minute at a fixed vaporizer setting of 8 at a fresh gas flow of 4 L/min and further increase from 1-1.5 MAC can be reached within 1 minute at a fresh gas flow of 2 L/min. Wash-out was found likewise flow dependent, but the time to reach a zero end-tidal concentration plateaued at 7.5 L/min.
This is a test model study. The research does not involve human participants and/or animals, and thus no informed consent has been requested. The set-up is entirely experimental and no human or animals have been exposed to anaesthetics, and thus no ethical review board assessment has been considered necessary.
Dataset 1: Raw data from the wash-in increase of Et-sevoflurane at fixed fresh gas flow and vaporiser setting. doi, 10.5256/f1000research.11255.d156064x
Dataset 2: Raw data from the wash-out of Et-sevoflurane at zero vaporiser setting and increasing fresh gas flow. doi, 10.5256/f1000research.11255.d156065xi
All authors have contributed equal to study design, set up, conduct of experiments, analysis, compilation, and preparation of manuscript
No competing interests were disclosed. Jan Jakobsson, have however previously received research grants for previous research activities from Maquet, Abbott, Baxter, MSD, Phitzer, Nycomed, PhaseIn, Grunenthal. He has been lecturing and taken part in advisory board activities for Maquet, Abbott, Baxter, MSD, Phitzer, Nycomed, PhaseIn, Masimo, Grunenthal. He has a paid consult agreement with Linde Healthcare as safety physician.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 26 Apr 17 |
read | read | read |
|
Version 1 29 Mar 17 |
read | read | |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)