Keywords
Nanopore sequencing, de novo genome assembly, wild type yeasts, ethanologenic, Candida, Cyberlindera
This article is included in the Nanopore Analysis gateway.
Nanopore sequencing, de novo genome assembly, wild type yeasts, ethanologenic, Candida, Cyberlindera
With the development of robust second generation bioethanol processes, next to the use of highly engineered Saccharomyces cerevisiae strains1,2, non-classical ethanologenic yeasts are also being considered as production organisms3,4. In particular, aspects concerning the ability to use both C6 and C5 C-sources and feedstock derived inhibitor resistance have been identified as important for the industrial applicability of different production hosts3. In our previous studies we have identified a novel ethanologenic yeast, Wickerhamomyces anomala, as a potential candidate3. Based on this research, a further screen for alternative yeast species was initiated (Punt and Omer, unpublished study) Here we describe the isolation and genomic characterization of one of these new isolates, which was typed as Candida vartiovaarae based on ribosomal RNA analysis.
With the arrival of next generation sequencing and the assemblers that can use this type of sequencing data, whole genome shotgun sequencing of completely novel organisms has become affordable and accessible. As a result, a wealth of genomic information has become available to the scientific community leading to many important discoveries. While generating whole draft genomes has become accessible, these genomes are often fragmented due to the nature of these short read technologies5. Assembling the short read data into large contigs proved to be difficult because the short reads do not contain the information to span repeated structures in the genome. Approaches to sequence the ends of larger fragments partially mitigated this problem6.
The new long read platforms from Pacific Biosciences and Oxford Nanopore Technologies made it possible to obtain reads that span many kilobases7. Assemblies using this type of data are often more contiguous than assemblies based on short read data8,9.
We have employed the Oxford Nanopore Technologies MinIONTM device to sequence genomic DNA from the isolated Candida vartiovaarae strain. The same DNA was also used to prepare a paired end library for sequencing on the Illumina HiSeq2500. The sequence data were used in various assemblers to obtain the best assemblies.
In our previous research3, a screening approach was developed to select for potential ethanologens using selective growth on industrial feedstock hydrolysates. Based on this approach, a previously identified microflora from grass silage was screened for growth on different hydrolysates from both woody and cereal residues. From this microflora, a strain was isolated (DDNA#1) after selection on a growth medium consisting of 10% acid-pretreated corn stover hydrolysate, which was shown to be most restrictive in growth due to the presence of relatively high amounts of furanic inhibitors.
Cells were grown at 30°C on plates with YNB (without amino acids) medium supplemented with 0.5% glucose. Cells were scraped from plates and resuspended in 5 ml TE. High MW chromosomal DNA was isolated from yeast isolate DNA#1 and Saccharomyces cerevisiae S288C using a Genomic-tip 100/G column, according to the manufacturer’s instructions (Qiagen).
To isolate intact chromosomal DNA from DDNA#1, a BioRad CHEF Genomic DNA Plug Kit was used. Briefly, yeast cells were treated with lyticase and the resulting spheroplasts were embedded in low melting point agarose. After incubation with RNase A and Proteinase K, the agarose plugs were thoroughly washed in TE. The DNA in the agarose plugs was separated on a 0.88% agarose gel in 1xTAE buffer on a Bio-Rad CHEF DRII system. The DNA was separated in four subsequent 12 hour runs at 3V/cm; run one and two used a constant switching time of 500 seconds, and in run three and four the switching time increased from 60 seconds to 120 seconds. The gel was afterwards stained with ethidium bromide and imaged.
High molecular weight DNA from both DDNA#1 and Saccharomyces cerevisiae S288C was sheared using a nebulizer (Life Technologies). The sheared DNA was used to make genomic DNA libraries using the TruseqTM DNA sample preparation kit, according to the manufacturer’s instructions (Illumina Inc.). In the size selection step, a band of 330–350 bp was cut out of the gel to obtain an insert length of ~270 bp. From the resulting libraries, 4.5 million fragments were sequenced in paired end reads with a read length of 150 nt on an Illumina HiSeq2500, according to the manufacturer’s instructions. The HiSeq control software (HCS) and real time analysis (RTA) software, versions were 2.2.38 and 1.18.61, respectively, were used.
The genomic DNA was sequenced using nanopore sequencing technology. First the DNA was sequenced on R7.3 Flow Cells. Subsequently, multiple R9 and R9.4 Flow Cells were used to sequence the DNA. For R7.3 sequencing runs, we prepared the library using the SQK-MAP006 kit from Oxford Nanopore Technologies. In short, high molecular weight DNA was sheared with a g-TUBE (Covaris) to an average fragment length of 20 kbp. The sheared DNA was repaired using the FFPE Repair Mix, according to the manufacturer’s instructions (New England Biolabs). After cleaning the DNA with using an extraction process, using a ratio of 0.4:1 Ampure XP beads (Beckman Coulter) to DNA, the DNA ends were polished and an A overhang was added with the NEBNext End Prep Module (New England Biolabs). Then, prior to ligation, the DNA was again cleaned with an extraction using a ratio of 1:1 Ampure XP beads to DNA. The adaptor and hairpin adapter were ligated using Blunt/TA Ligase Master Mix (New England Biolabs). The final library was prepared by cleaning the ligation mix using MyOne C1 beads (Invitrogen).
To prepare 2D libraries for R9 sequencing runs, we used the SQK-NSK007 kit from Oxford Nanopore Technologies. The procedure to prepare a library with this kit is largely the same as with the SQK-MAP006 kit. 1D library preparation was done with the SQK-RAD001 kit from Oxford Nanopore Technologies. In short, high molecular weight DNA was tagmented with a transposase. The final library was prepared by ligation of the sequencing adapters to the tagmented fragments using the Blunt/TA Ligase Master Mix (New England Biolabs).
The prepared libraries were loaded on the MinION flow cell, which was docked on the MinION device. The MinKNOW software (version 0.50.2.15 for SQK-MAP006 libraries and version 1.0.5 for SQK-NSK007 and SQK-RAD001 libraries) was used to control the sequencing process and the read files were uploaded to the cloud based Metrichor EPI2ME platform for base calling. Base called reads were downloaded for further processing and assembly.
The sequence data from the Illumina platform was assembled using the Spades assembler (version 3.6.0), either alone or in combination with the nanopore data.
From the base called read files produced by the Metrichor EPI2ME platform, a sequence file in fasta format was extracted using the R-package poRe v0.1710. For the assembly of the nanopore data, Canu v1.3 was used11. After assembly, the resulting contigs were polished with the short read data using PILON v1.1812. The sequencing data has been submitted to the European Nucleotide Archive and can be accessed at http://www.ebi.ac.uk/ena/data/view/PRJEB19912.
A k-mer count analysis was done using Jellyfish (version 2.2.6)13 on the Illumina data. From the paired end reads, only the first read was truncated to 100 bp to avoid the lower quality part of the read. The second read was omitted from this analysis to avoid counting overlapping k-mers. Different k-mer sizes were used ranging from k=17 to 23. After converting the k-mer counts into a histogram format, this file was analyzed using the Genomescope tool, available at http://qb.cshl.edu/genomescope/ and https://github.com/schatzlab/genomescope.
From 26S ribosomal RNA sequences available in the nucleotide database, Chen et al.14 have constructed a phylogenetic tree. The closest relative for which whole genome sequences are available is Cyberlindnera jadinii. To compare our draft genome assembly to this yeast species, we retrieved assemblies of two Cyberlindnera jadinii strains, namely NBRC 0988 (GenBank accession number, DG000077.1) and CBS1600 (GenBank accession number, CDQK00000000.1). We also used Saccharomyce cerevisiae S288C (GenBank accession number, GCA_000146045.2) in this comparison. We aligned those assemblies to the corrected draft assembly of our strain using MUMmer’s alignment generator NUCmer (version 3.1)15. NUCmer’s output was filtered with delta-filter, and the filtered results parsed to MUMmerplot, generating full-genome visualization between the pairs of different yeast species.
Reads generated on the Illumina platform were aligned to the published Candida vartiovaarae mitochondrial genome (Genbank accession number, KC993190.1) using Bowtie2 (version 2.2.5). Reads generated on the MinION platform were aligned using BWA-mem (version 0.7.15) with -x ont2d settings. Resulting bam files were sorted and viewed in IGV viewer (version 2.3).
From a screen on 10% acid-pretreated corn stover hydrolysate, about 70 individual clones were obtained, only five of which were able to grow well on purely synthetic YNB-based medium. To determine the taxonomic status of these clones, chromosomal DNA was isolated and used for PCR amplification of the ribosomal ITS sequence using ITS specific primers (ITS1 and ITS416).
BLAST analysis of these ITS sequences of all 5 isolates revealed a 100% identity to Candida vartiovaarae (Torulopsis vartiovaarae: NCBI accession number KY102493)
All five isolates were grown on different C-sources and showed growth on glucose, mannose, cellobiose, xylose and glycerol, while growth on L-arabinose was variable. No significant growth was found on galactose and rhamnose. Good growth (on glucose) occurred between 20–30°C, at pH3-7 (optimum 25°C, pH4-5). Based on the results, we concluded that all five isolates originated from a single source in the grass silage sample. Subsequent experiments were therefore carried out with a single isolate now named DDNA#1.
We took three approaches to assemble the genome of DDNA#1. The first approach used only short reads produced by the Illumina platform. After merging the paired end reads we obtained 1.08 Gbp of ~240 bp reads. The genome sequence that we obtained using the Spades assembler17 showed a very fragmented assembly that consisted of 14,764 contigs. The N50 of this assembly was only 2.2 kbp, possibly due to a high level of SNPs. We also assembled Saccharomyces cerevisiae S288C using a similar short read dataset that was made and sequenced in parallel. Here we obtained an assembly that consisted of 768 contigs with a longer N50 of 124 kbp. In the second approach, we used the Spades assembler to make a hybrid assembly by combining the short read data set and the corrected long reads that were produced by the Canu assembler11. From the original 2.05 Gbp nanopore sequence data with an average read length of 7.5 kbp, 389 Mbp was left after correction by Canu. This corrected dataset had an average read length of 7.9 kbp. This hybrid assembly consisted of 1904 contigs with an N50 of 255 kbp. As a third approach, we only used the long read data set and let the Canu assembler correct the longest reads with the shorter reads and then attempt an assembly. In this assembly we obtained 61 contigs with a N50 of 455 kbp (Table 1). It is clear from these results that using the long read data set alone produced the most contiguous assembly, as has been shown previously8,9.
We also used the nanopore datasets made with the R7.3 and R9 chemistry separately in the Canu assembler. The most notable difference between these assemblies is found in the mitochondrial genome. Only 16 kbp of this 33 kbp genome could be assembled with the R7.3 data, whereas the R9 assembly contained the complete mitochondrial genome (NCBI reference sequence, NC_022164.1). The mitochondrial genome has a very low GC content (21%) and in the extragenic regions more A and T homopolymers are found. Very few R7.3 reads mapped to this region, but in the R9 dataset there are many more reads that represent this region (Figure 1). It has been shown that the R7.3 data especially has a bias against A and T homopolymers. This bias is reduced in R9, but not completely absent18,19. Even after correction of the long reads and assembly in Canu the contig sequences still contain errors11. We have used PILON12 and the complementary Illumina data from this strain to correct the assembled contigs. This led to a minor increase in size of the assembly.

Reads from both the Illumina, and the nanopore platform were aligned to the Candida vartiovaarae mitochondrial genome (Genbank accession number, KC993190.1) to show the difference in coverage between the different platforms and chemistry versions.
The Illumina sequence data of our DDNA#1 isolate were submitted to the Genomescope13 software package to analyze the k-mer count distribution, using k-mer size = 19 at an average coverage of 28.0x (Figure 2). The ‘haploid’ genome is predicted to contribute to the most abundant fraction, which corresponds with the second peak (dotted line) in the plot (Figure 2A). The first peak corresponds to sequence occurring exactly half as frequently as the main peak, so these are plausibly haplotypes. Due to the nature of k-mer counting, this peak often appears higher than the main peak, because a single SNP will affect all k-mers overlapping that position. The first two peaks contain about 10 Mbp of sequence. Additional peaks at higher coverage indicate duplications and repetitive DNA that are quite abundant, but correspond with less sequence than the second peak. Genomescope estimated a haploid genome size of between 12.00 and 12.01 Mbp. Additionally, Genomescope revealed 3.6% variety across the entire genome indicating that the genome of C. vartiovaarae has strong heterozygous properties (Figure 2B). A likely possibility is that areas in the genome are replicated and slightly diverged in sequence. This could also explain why we see a large tail of repeated k-mers (Figure 2A). It could also explain why our assembly still remained fragmented despite the relatively large amount of nanopore data that was used in the assembly.
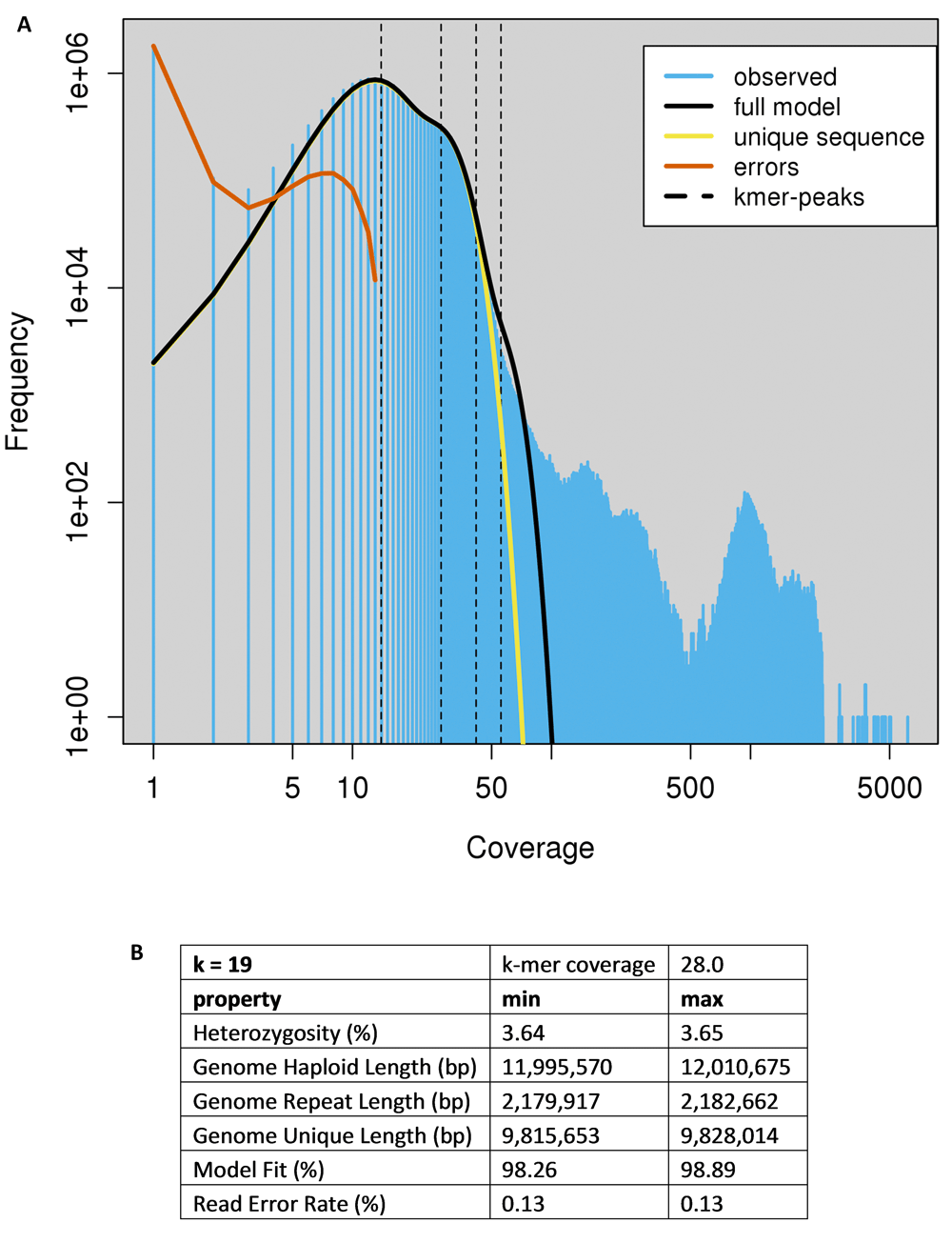
Genomescope attempts to find k-mer count peaks, low and high coverage peaks indicating hetero- and homozygosity. (A) We find ~13× and ~28× coverage for hetero- and homozygous fractions in our dataset. Exact peak positions are determined with a log transformation. Evaluating the slope between coverage points reveals the peak positions indicating hetero- and homozygosity, for lower and higher coverage, respectively. (B) Table showing the most important metrics from this k-mer analysis.
As a further means to validate our assembled contigs and determine if they match the actual chromosome length, we have separated the chromosomes on an agarose gel using pulsed field gel electrophoresis. The gel image in Figure 3 shows five bands that represent the chromosomes of this yeast strain. The smallest band has a length that corresponds to the length of the mitochondrial genome (33 kbp). Additional fragments of 450, 1200, and 1500 kbp are also found. The intensity of the band that runs above the 2200 kbp marker band suggests that it actually contains more than one distinct fragment. To make the genome size fit to the estimate derived from the assembly and k-mer analysis (~12.5 mbp), three ~3 Mbp chromosomes should be postulated. The uncertainty in chromosome size estimate based on pulsed field electrophoresis gels is high because of the large chromosome size and the fact that it is difficult to determine if more than one fragment is present in the gel at a given position. Our conclusion that the top band represents three or more chromosomes is in agreement with the genome sequences of two related C. jadinii strains, namely CBS1600 and NBRC 0988.
We have compared the assembled contigs of our C. vartiovaarae isolate DDNA#1 strain to yeast genome sequences that are already deposited in the nucleotide database. Comparison of our yeast strain with the well characterized S. cerevisiae assembly showed negligible genomic similarity (Figure 4A). From 26S ribosomal RNA sequences available in the nucleotide database, Chen et al.14 have constructed a phylogenetic tree. The closest relatives for which whole genome sequences are available are C. jadinii strains CBS1600 and NBRC 0988. An initial comparison between CBS1600 and NBRC 0988 revealed that these two strains show high homology (Figure 4B). The genomic similarity between our strain and C. jadinii strains CBS1600 and NBRC 0988 is much lower (Figures 4C and D). In conclusion, these data show that wild type yeast strains are very heterogeneous, despite a high similarity based on ribosomal RNA ITS sequences. Therefore, the data suggest that nanopore sequencing is an essential new tool to classify yeast strains. Of course, the nanopore sequence data in combination with other sequencing technologies is highly useful for accurate annotation of all genes in the genome.
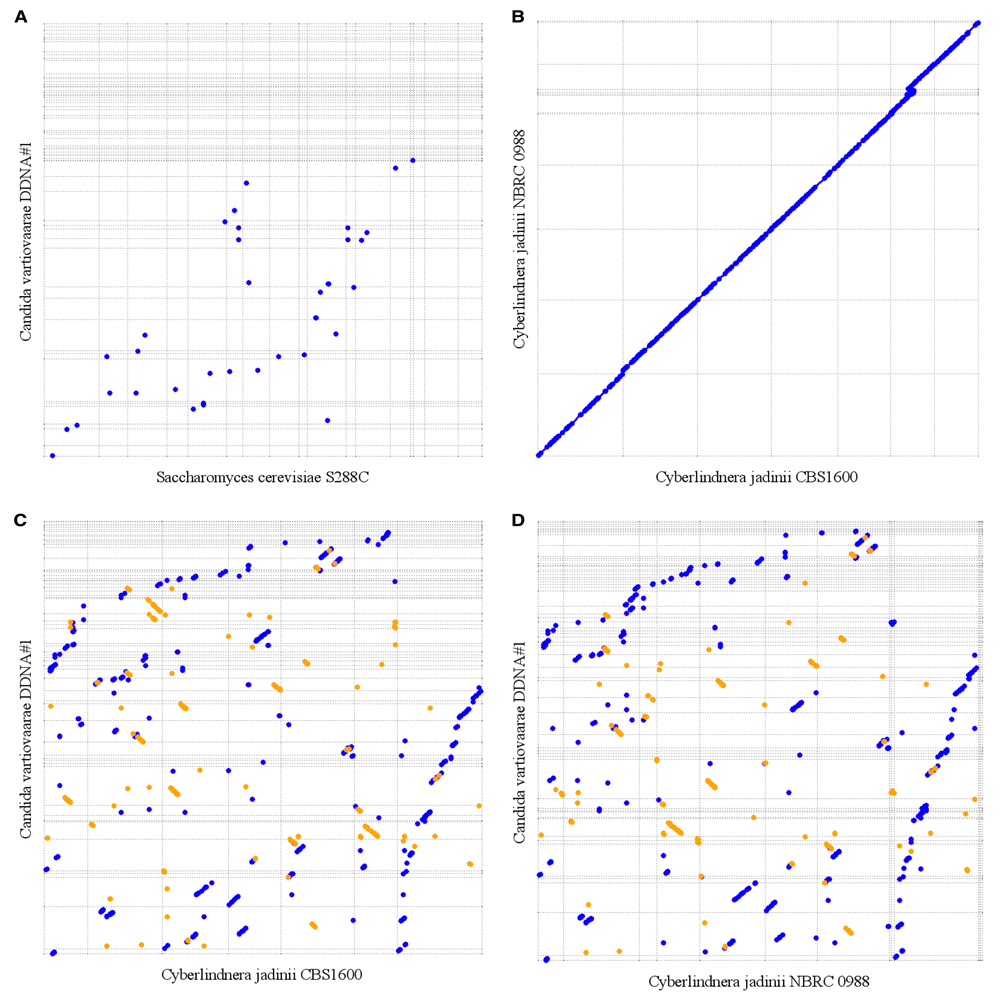
Dashed lines indicate contigs (start and stop positions) and the area between dashed lines indicates the contig size. Blue and orange dots are hits in reverse and forward orientation, respectively. Diagonal lines indicate sequence and synteny conservation across species. (A) Comparison between Saccharomyces cerevisiae S288c (horizontal axis) and Candida vartiovaarae isolate DDNA#1 (vertical axis). (B) Comparison between Cyberlindnera jadinii strains CBS1600 (horizontal axis) and NBRC 0988 (vertical axis). (C) Comparison between Candida vartiovaarae isolate DDNA#1 (vertical axis) and Cyberlindnera jadinii strain CBS1600 (horizontal axis). (D) Comparison between Candida vartiovaarae isolate DDNA#1 (vertical axis) and Cyberlindnera jadinii strain NBRC 0988 (horizontal axis).
HPS conceived the study. PJP, HPS, HJJ, and RPD designed the experiments. HJJ, RJLFL, PvH, TO, and SS performed the experiments. HJJ, ML, and CVH contributed to the data analysis. HJJ, RPD, and HPS prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
HJJ and CVH are members of the Nanopore Community, and have previously received flow cells free of charge, as well as travel expense reimbursements from Oxford Nanopore Technologies.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: We declare that we have no competing interests; however we should mention that we are part of the MinION® Access Programme (MAP) and JMA received travel and accommodation expenses to speak at Oxford Nanopore Technologies conferences.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 03 Aug 18 |
read | read | read | |
|
Version 1 03 May 17 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)