Keywords
antiviral innate immunity, Cauliflower mosaic virus, error catastrophe, hypermutagenesis, mutational spectrum, plant-virus interaction, pararetrovirus, virus evolution,
antiviral innate immunity, Cauliflower mosaic virus, error catastrophe, hypermutagenesis, mutational spectrum, plant-virus interaction, pararetrovirus, virus evolution,
The human APOBEC (apolipoprotein B mRNA editing catalytic polypeptide-like) family includes enzymes that catalyze the hydrolytic deamination of cytidine to uridine or deoxycytidine to deoxyuridine. This family is composed of eleven known members: APOBEC1, APOBEC2, APOBEC3 (further classified as A3A to A3H), APOBEC4, and AID (activation induced deaminase). APOBEC proteins are associated with several functions involving editing of DNA or RNA (reviewed by Smith et al1). APOBEC1 mediates deamination of cytidine at position 6666 of apolipoprotein B mRNA, resulting in the introduction of a premature stop codon and the production of the short form of the protein2–4. APOBEC2 is essential for muscle tissue development5. APOBEC4 has no ascribed function so far6. AID deaminates genomic ssDNA of B cells, initiating immunoglobulin somatic hypermutation and class switch processes7–9. Most notably, APOBEC3 enzymes participate in innate immunity against retroviruses and endogenous retroelements10–12. Sheehy et al. demonstrated that A3G also plays a role in immunity against human immunodeficiency virus type 1 (HIV-1)13. For its antiviral role, A3G is packaged along with viral RNA14. Upon infection of target cells and during the reverse transcription process, A3G deaminates the cytosine residues of the nascent first retroviral DNA strand into uraciles. The resulting uracil residues serve as templates for the incorporation of adenine, which at the end result in strand-specific C/G to T/A transitions and loss of infectivity through lethal mutagenesis15–19. On the other hand, sub-lethal mutagenic activity of APOBEC3 proteins may end up being an additional source for HIV-1 genetic diversity, hence bolstering its evolvability20–22. APOBEC3 proteins have been shown to inhibit other retroviruses (simian immunodeficiency virus23, equine infectious anemia virus24, foamy virus25, human T-cell leukemia virus26, and murine leukemia virus27), pararetroviruses (hepatitis B virus28) and DNA viruses (herpes simplex virus 129,30, Epstein-Barr virus30, HSV-1 and EBV respectively, and human papillomavirus31). In the cases of HSV-1 and EBV, the antiviral role of deaminases has not yet been demonstrated30. Evidence also exists that A3G significantly interferes with negative-sense RNA viruses lacking a DNA replicative phase32. For example, the transcription and protein accumulation of measles virus, mumps virus and respiratory syncytial virus (RSV) was reduced 50–70%, whereas the frequency of C/G to U/A mutations was ∼4-fold increased32. In contrast, A3G plays no antiviral activity against influenza A virus despite being highly induced in infected cells as part of a general IFN-β response to infection33,34.
Human APOBEC belongs to a superfamily of polynucleotide cytidine and deoxycytidine deaminases distributed throughout the biological world35. All family members contain a zinc finger domain (CDD), identifiable by the signature (H/C)-x-E-x25-30P-C-x-x-C. Plants are not an exception and, for example, the Arabidopsis thaliana genome encodes nine putative cytidine deaminases (with genes named AtCDA1 to AtCDA9). Whilst the AtCDA1 gene is located in chromosome II, the other eight genes are located in chromosome IV. In the case of rice and other monocots, only one CDA has been identified35. Interestingly, this CDA expression was highly induced as part of the general stress response of rice against infection of the fungal pathogen Magnaporthe grisea, resulting in an excess of A to G and U to C mutations in defense-related genes36. Edited dsRNAs might be retained in the nucleus and degraded, generating miRNAs and siRNAs37. Given the relevance of deamination as an antiviral innate response in animals, we sought first to determine whether any of the AtCDA proteins encoded by plants can participate in deaminating the genome of the pararetrovirus, cauliflower mosaic virus (CaMV; genus Caulimovirus, family Caulimoviridae) and, second, we sought to explore whether this deamination may negatively impact viral infection. We hypothesize that deamination may take place mainly at the reverse transcription step. The CaMV genome is constituted by a single molecule of circular double-stranded DNA of 8 kbp38. The DNA of CaMV has three discontinuities, Δ1 in the negative-sense strand (or a strand), and Δ2 and Δ3 in the positive-sense strand (yielding the b and g strands). In short, the replication cycle of CaMV is as follows38: in the nucleus of the infected cell, the a strand is transcribed into 35S RNA, with terminal repeats, that migrates to the cytoplasm. Priming of the 35S RNA occurs by the annealing of the 3’ end of tRNAmet to the primer-binding site (PBS) sequence, leading to the synthesis of the DNA a strand by the virus’ reverse transcriptase. Then, the RNA in the heteroduplex is degraded by the virus’ RNaseH activity, leaving purine-rich regions that act as primers for the synthesis of the positive-sense DNA b and g strands.
Our results show that AtCDA1 significantly increases the number of G to A mutations in vivo, and that there is a negative correlation between the amount of AtCDA1 mRNA present in the cell and the load reached by CaMV, suggesting that deamination of viral genomes may also constitute a significant antiviral mechanism in plants.
AtCDAs cDNAs were cloned under the 35S promoter in a pBIN61 vector39. N. bigelovii plants were inoculated with CaMV virions purified from Brassica rapa plants40 previously infected with the clone pCaMVW26041. Symptomatic leafs were agroinfiltrated39 with one of the nine AtCDAs and with the empty vector pBIN61, each on one half of the leaf. Samples were collected three days post-agroinfiltration.
The design and cloning of the artificial micro-RNA (amiR) able to simultaneously suppress the expression of AtCDAs 1, 2, 3, 4, 7, and 8 was performed as described in ref. 42. The amiRNA was cloned under the control of Aspergillus nidulans ethanol regulon43,44 and used to transform A. thaliana by the floral dip method45. By doing so, we obtained the transgenic line amiR1-6-3. One-month-old seedlings of transgenic and wild-type A. thaliana were treated with 2% ethanol (or water for the control groups) three times every four days. Three days after the third treatment, plants were inoculated with the infectious clone pCaMVW260 as described in ref. 41. Infections were established by applying 1.31×1011 molecules of pCaMVW260 to each of three leaves per plant. Subsequently, plants were subjected to two additional treatments with 2% ethanol (or water) one and five days post-infection. Finally, samples were taken eight days after inoculation and handled as previously described46. For each genotype (transgenic or wild-type) and treatment (ethanol or water) combination, 22 plants were analyzed.
CaMV genomic DNA was purified using DNeasy Plant Mini Kit (Qiagen) according to manufacturer’s instructions. For detection of edited genomes 3D-PCR was performed using primers HCa8Fdeg and HCa8Rdeg. PCRs were performed in a Mastercycler® (Eppendorf) at denaturation temperatures 82.1 °C, 82.9 °C, 83.9 °C, and 85.0 °C. PCR products obtained with the lowest denaturation temperature were cloned in pUC19 vector (Fermentas), transformed in Escherichia coli DH5α and sent to GenoScreen (Lille, France) for sequencing.
Total RNA was extracted from A. thaliana plants using the RNeasy® Plant Mini Kit (Qiagen), according to manufacturer’s instructions. AtCDA1 specific primers qCDA1-F and qCDA1-R were designed using Primer Express software (Applied Biosystems). RT-qPCR reactions were performed using the One Step SYBR PrimeScript RT-PCR Kit II (Takara). Amplification, data acquisition and analysis were carried out using an Applied Biosystems Prism 7500 sequence detection system. All quantifications were performed using the standard curve method. To quantify AtCDA1 mRNA, a full-ORF runoff transcript was synthetized with T7 RNA polymerase (Roche) using as template a PCR product obtained from cloned AtCDA1 and primers T7-CDA1F and qCDA1-R. CaMV qPCR quantitation was performed as described in ref. 46.
To test the mutagenic activity of A. thaliana CDAs, nine N. bigelovii plants were inoculated with CaMV. After systemic infection was established, we performed transient AtCDA overexpression experiments. To do so, the same leaf was agroinfiltrated twice; one half of the leaf was infiltrated with one of the nine AtCDA genes and the other half of the leaf was infiltrated with the empty vector. This test was done for all nine AtCDA genes in different plants. The presence of AtCDA mRNAs was verified by RT-PCR from DNase-treated RNA extracts. DNA was extracted from agroinfiltrated areas for 3D-PCR amplification of a 229 bp fragment in the ORF VII of CaMV. 3D-PCR uses a gradient of low denaturation temperatures during PCR to identify the lowest one, which potentially allows differential amplification of A/T rich hypermutated genomes47. There were no differences in the lowest denaturation temperature that could result in differential amplification of controls and the AtCDA-agroinfiltrated samples, suggesting that hypermutated genomes should be at low frequency, if present at all.
PCR products obtained at the lowest denaturation temperature were cloned and sequenced. In a preliminary experiment, we sequenced 25 clones from each AtCDA/negative control pair (Supplementary Table S1). At least one G to A transition was detected in clones from areas infiltrated with AtCDA1, AtCDA2 and AtCDA9 genes. For these three genes, we further increased the number of sequenced clones up to 106. The CaMV mutant spectra was significantly different between plants overexpressing AtCDA1 and their respective negative controls (Figure 1a: χ2 = 25.760, 7 d.f., P = 0.001). This difference was entirely driven by the 471.43% increase in G to A transitions observed in the plants overexpressing AtCDA1. A thorough inspection of alignments showed that most of the G to A mutations (65.6%) detected in the different samples were located at the nucleotide position 181 (Supplementary Table S1). By contrast, no overall difference existed between the mutant spectra of CaMV populations replicating in plants overexpressing AtCDA2 (Figure 1b: χ2 = 8.944, 6 d.f., P = 0.177) or AtCDA9 (Figure 1c: χ2 = 6.539, 8 d.f., P = 0.587) and their respective controls. Consistently, the mutant spectra from the three AtCDA-overexpressed samples were significantly heterogeneous (χ2 = 41.063, 16 d.f., P = 0.001), again due to the enrichment in G to A transitions observed in the case of AtCDA1. By contrast, the three independent control inoculation experiments showed homogeneous mutant spectra for CaMV (χ2 = 14.605, 18 d.f., P = 0.689), undistinguishable from the mutant spectra previously reported for natural isolates of this virus48. The consistency of the mutant spectra observed for the three control experiments and for a natural isolate of the virus suggests that in absence of a perturbation such as the overexpression of AtCDA1, the CaMV mutant spectrum is rather stable.
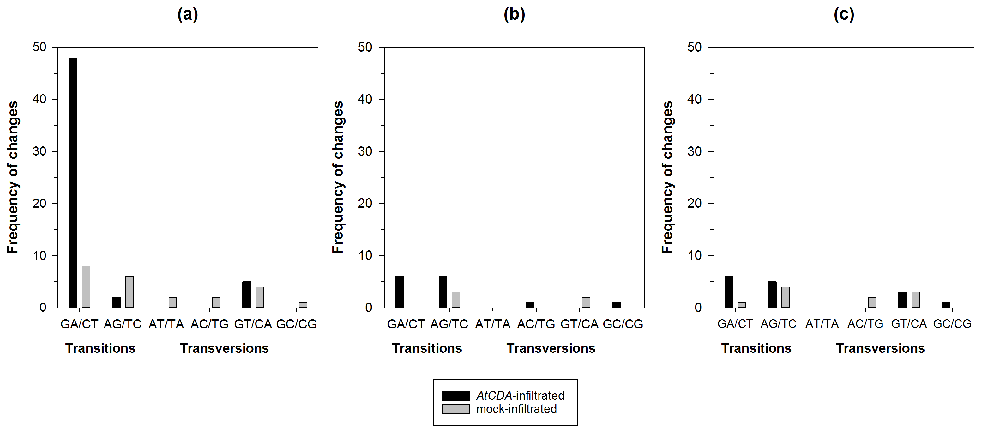
(a) AtCDA1, (b) AtCDA2 and (c) AtCDA9. The pBIN61 empty vector was agroinfiltrated in the same leaves than their corresponding AtCDAs (mock). For each sample 20,034 nucleotides were sequenced.
We conclude that overexpressing the AtCDA1 gene results in a significant shift in CaMV genome composition towards G to A mutations, as expected from cytidine deaminase hypermutagenic activity.
To test the effects of suppressing the expression of AtCDA on viral accumulation we produced a transgenic line of A. thaliana Col-0, named amiR1-6-3. This line was stably transformed with an amiR, controlled by the A. nidulans ethanol regulon to achieve ethanol-triggered RNAi-mediated simultaneous suppression of AtCDAs 1, 2, 3, 4, 7, and 8 expression. Transgenic and wild-type plants were subjected to periodical treatment with 2% ethanol (or water for the control groups). Subsequently, plants were inoculated with the infectious clone pCaMVW260 that expresses the genome of CaMV. Samples were taken eight days after inoculation and AtCDA1 mRNA and CaMV viral load were quantified by real time RT-qPCR and qPCR, respectively, in the same samples. For each genotype and/or treatment, 22 plants were analyzed.
The expression of AtCDA1 mRNA depended on the plant genotype (Figure 2a; GLM: χ2 = 28.085, 1 d.f., P < 0.001) as well as on the interaction of plant genotype and treatment (χ2 = 26.037, 1 d.f., P < 0.001), suggesting a differential accumulation of AtCDA1 mRNA on each plant genotype depending on the amiR1-6-3 induction state. Ethanol treatment reduced the amount of AtCDA1 mRNA by 24.01% in transgenic plants, proving that triggering the expression of the amiR1-6-3 significantly and efficiently silences the expression of AtCDA1. Unexpectedly, the effect was the opposite in wild-type plants, for which we observed 23.76% increase in AtCDA1 mRNA accumulation (Figure 2a) upon treatment with ethanol. This increase in expression of AtCDA1 in wild-type plants after ethanol treatment and the underlying mechanisms certainly deserve to be investigated further. However, for the purpose of this study, its relevance is that it may increase the number of G to A mutations in the CaMV genome, thus making the antiviral effect stronger to some extent.
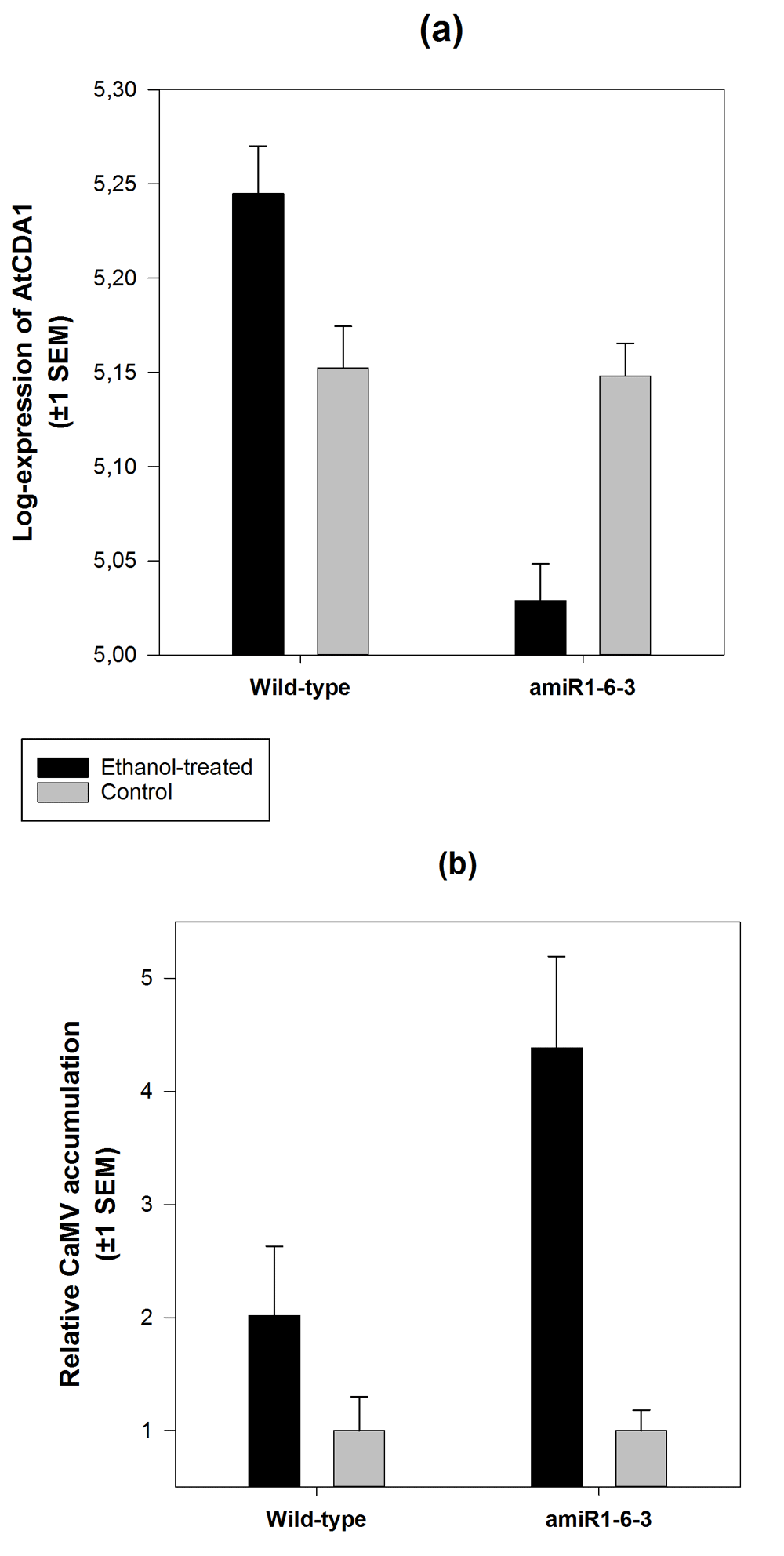
(a) Number of AtCDA1 mRNA molecules/80 ng total RNA quantified by RT-qPCR using the standard curve method for absolute quantification. (b) Number of CaMV genomes/80 ng total DNA. For each block of plants (wild-type and amiR1-6-3), values were normalized to the average number of genomes estimated in the corresponding water-treated (control) plants.
More interestingly, the relative accumulation of CaMV in ethanol-treated plants was significantly different, depending on the plant genotype being infected (Figure 2b; Mann-Whitney U test, P = 0.002): silencing the AtCDA1 gene bolstered CaMV accumulation to 103.10% compared to the accumulation observed in wild-type plants. Furthermore, there was a significant negative correlation between the number of molecules of AtCDA1 mRNA and viral load (partial correlation coefficient controlling for treatment: r = –0.299, 86 d.f., P = 0.005).
Given the significant increase of viral load in plants with lower levels of AtCDA1 mRNA, we sought the molecular signature of deamination in transgenic plants. For this, we selected three biological replicates from each treatment group (ethanol or control) and sequenced between 39–45 clones of the CaMV fragment from each replicate. As shown in Figure 3, silencing of the AtCDA1 gene affects the composition of CaMV mutant spectrum by reducing the number of G to A transitions by 69.23%. Nevertheless, overall, both mutational spectra were not significantly different (Figure 3: χ2 = 9.108, 6 d.f., P = 0.168), prompting caution against making a definite conclusion on the role of deamination in the observed increase in CaMV accumulation.
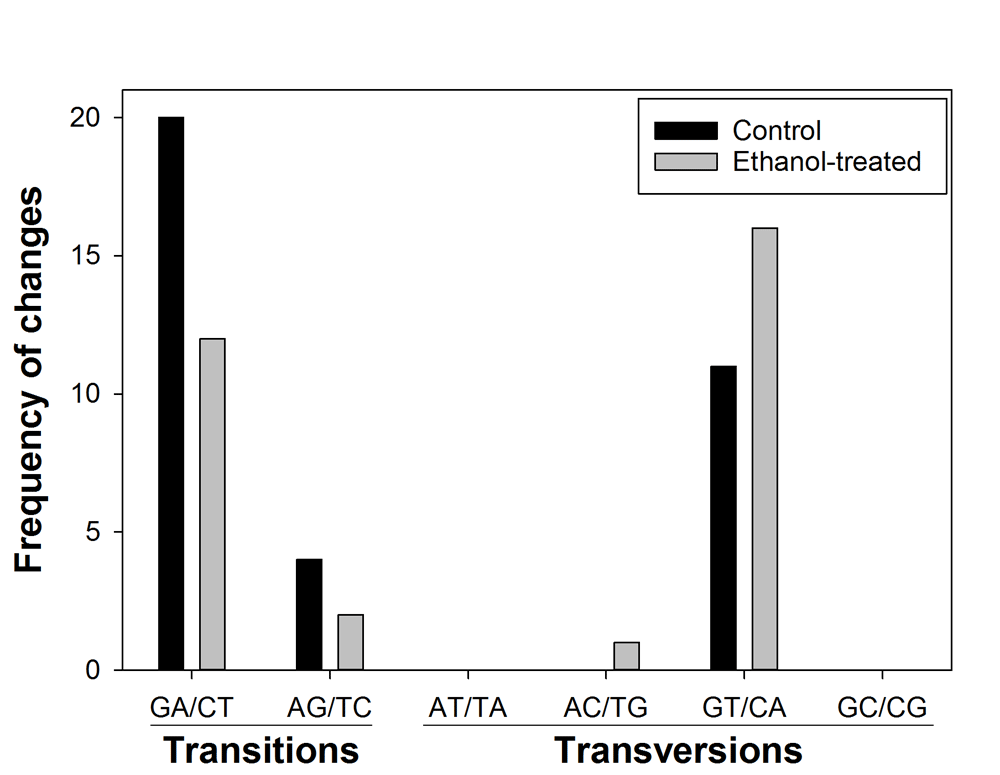
The number of nucleotides sequenced was 23,436 for control and 24,003 for ethanol-treated plants. Ethanol-treated plants turn on the expression of amiR1-6-3 that was designed to silence the expression of the AtCDA1 gene.
We conclude that suppressing the expression of the AtCDAs 1, 2, 3, 4, 7, and 8 significantly reduces the accumulation of CaMV. However, the characterization of the mutant spectrum of the same CaMV populations provides no strong enough support to the cytidine deamination hypothesis.
Lethal mutagenesis through deamination of RNA/DNA by cytidine deaminases has been proven to work as an antiviral mechanism against retroviruses16–19,23–27, and some DNA28–31 and RNA32 viruses infecting mammals. Our results show that the A. thaliana CDA1 gene has some degree of mutagenic activity on the pararetrovirus CaMV genome. Moreover, simultaneously suppressing the expression of a subset of AtCDAs, including AtCDA1, increased CaMV load, strongly suggesting an antiviral role for AtCDAs.
Our data show that AtCDAs probably restrict CaMV replication through a process similar to the restriction of HIV-1 by APOBEC3. CaMV replicates in the cytoplasm by reverse transcription using the positive-sense 35S RNA as template. As for HIV-1, the first strand negative-sense cDNA could be deaminated during reverse transcription, transforming deoxycytidine into deoxyuridine. Then, when the positive-sense strand is produced, an A is incorporated instead of a G, increasing the proportion of G to A mutations. In the case of HIV-1, this G to A mutational bias is explained by A3G and A3H specificity for single negative stranded DNA: during HIV-1 replication, C to G transitions are rare and restricted to the PBS site and U3 regions in the 5’ long terminal repeat, where positive-stranded DNA is predicted to become transiently single stranded49. Similarly, during CaMV replication the negative strand remains single stranded, while the positive is copied from it and remains double stranded50. Surprisingly, for AtCDA1, C to T mutations were also increased; the region studied here is close to the 5’ end of CaMV, which contains the PBS for negative-strand synthesis and the ssDNA discontinuity ∆1. The observed C to T transitions could reflect transient positive-stranded ssDNA in the 5’ terminal region during reverse transcription, nevertheless a different substrate specificity of A. thaliana CDAs cannot be ruled out.
Most of the G to A transitions detected in agroinfiltration experiments were located in the G at position 181. HIV-1 hypermutated genomes show mutational hot spots as well, which are due to preference of A3G and A3F for deamination of the third C in 5’-CCC (negative-strand) and 5’-GGC, respectively51,52. The context of the C complementary to G181 (5’-GGC) differs from what has been described for APOBEC3, suggesting that if AtCDAs had context preference, it would be different from the one described for A3G. However, given the low number of mutations found, we should be cautious when concluding whether AtCDAs have a possible sequence-context preference. Since our experiments were performed in vivo, negative selection is expected to purge genomes carrying deleterious mutations. This limitation could account for our failure to detect largely hypermutated genomes, and demonstrates the need for developing new selection-free assays to further characterize AtCDA-induced mutagenesis.
Although there is not a demonstrated correlation between the expression of APOBEC3 and mutational bias of viruses infecting mammals, caulimoviruses have an excess of G to A transitions in synonymous positions53. In A. thaliana plants, we found that silencing of AtCDA1 reduced the frequency of G to A transitions in the CaMV genome, suggesting a contribution of AtCDAs to the nucleotide bias found in caulimoviruses. The increased viral load in CDA-silenced A. thaliana plants strongly suggests that deamination of viral genomes may work as an antiviral mechanism in plants, leading to questions about how general this mechanism might be, and how it may contribute to viral evolution. Describing a new natural antiviral mechanism in plants opens new research avenues for the development of new durable control strategies.
All datasets that support the findings in this study are available at LabArchives with DOI: 10.6070/H4TD9VD5.
‘File Sequence_data_for_Figure_1.zip’ contains the FASTA files with the sequence data used to generate the mutational spectra shown in Figure 1.
‘Data_for_Figure_2a.xlsx’ contains the AtCDA1 expression data used to generate Figure 2a.
‘Data_for_Figure_2b.xlsx’ contains the CaMV accumulation data used to generate Figure 2b.
‘Sequence_data_for_Figure_3.zip’ contains the FASTA files with sequence data used to generate the mutational spectra shown in Figure 3.
SFE conceived the study, designed the experiments and analyzed the data. SM, JMC and AG-P performed the experiments and contributed to experimental design. SM, JMC and SFE wrote the paper. All authors revised and approved the manuscript.
This work was supported by the former spanish Ministerio de Ciencia e Innovación-FEDER grant BFU2009-06993 to SFE. JMC was supported by the CSIC JAE-doc program/Fondo Social Europeo. AG-P was supported by a grant for Scientific and Technical Activities and by grant P10-CVI-65651, both from Junta de Andalucía.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Francisca de la Iglesia (IBMCP-CSIC), Àngels Pròsper (IBMCP-CSIC) and Ana Cuadrado (IBMCP-CSIC) for excellent technical assistance, Miguel A. Blázquez (IBMCP-CSIC) for help in designing the amiR1-6-3 and generating the transgenic plants and Rémy Froissart (BGPI-CNRS) for providing the pCaMVW260 infectious clone.
Supplementary Table S1. Nucleotide substitutions detected in the overexpression experiments. For each of the nine infiltrated plants, the substitutions observed in the clonal sequences analyzed at the overexpressed (AtCDA1 to AtCDA9) and control (pBIN61-infiltrated) regions are shown. In some cases, a given substitution is present in several clonal sequences from the same sample and the number of times it appears is indicated between parentheses. Nucleotide positions are given according to CaMV isolate W260, GenBank accession JF809616.1.
Click here to access the data.
Supplementary Table S2. Nucleotide substitutions found in A. thaliana transgenic plants with or without inducing the expression of amiR3-1-9 that silences the expression of several AtCDAs. In some cases, a given substitution is present in several clonal sequences from the same sample and the number of times it appears is indicated between brackets. Nucleotide positions are given according to CaMV isolate W260, GenBank accession JF809616.1.
Click here to access the data.
Supplementary Table S3. Primers used in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant virus evolution
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Viral Evolutionary Ecology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 15 Jun 17 |
||
|
Version 1 03 May 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)