Keywords
Saccharomyces cerevisiae, Saccharomyces kudriavzevii, hybrids, cold stress, winemaking,
Saccharomyces cerevisiae, Saccharomyces kudriavzevii, hybrids, cold stress, winemaking,
In this version we included more discussion about the relation of ribosome recycling and the cold adaptation. Also we included that qPCR were tested for allele specificity and comparable efficiency.
See the authors' detailed response to the review by Matthias Sipiczki
See the authors' detailed response to the review by Samuel Marguerat
Wine yeasts are specialised organisms adapted to restrictive environmental conditions created by human technology. The most frequently isolated species in wine fermentations is Saccharomyces cerevisiae, but other species of the Saccharomyces genus and their interspecific hybrids are also present in the final wine fermentations. These species have attracted significant interest in the last years due to their potential in solving the main challenges the winemaking industry faces, such as the enhancement of aroma. There is a trend in winemaking to decrease fermentation temperatures, which improves the wine aromatic profile. The wine industry has yeasts that are commercialized as cryotolerant S. cerevisiae yeasts, however most of them do not show desirable fermentation performance at low temperatures (10–15°C).
Several studies have addressed the yeast cold stress adaptation topic. A transcriptomic analysis using QA23, a commercial S. cerevisiae wine-making strain, during low temperature industrial fermentations showed how the expression profiles at 25°C contrasted significantly with those at 13°C1. In particular, the expression of genes associated with cell growth, cell cycle and maintenance was lower in the exponential phase at 13°C than at 25°C, and those genes activated in the exponential phase of growth at 13°C were essentially involved in environmental stress response2.
Physiological and enological studies have suggested the potential benefits of the use of S. kudriavzevii in low temperature wine fermentations, and its cryotolerant nature3. In a study comparing transcriptomes of S. kudriavzevii and S. cerevisiae, both species showed up-regulation of genes related to translational machinery, although S. kudriavzevii presented an enhanced response compared to S. cerevisiae4. Tronchoni et al.4 postulated that this response could be the result of alterations in the stability of a functional RNA conformation related to a competing structure. This suggests adaptation to cold shock in S. kudriavzevii due to higher ribosome availability and enhanced translation efficiency.
In cold European regions S. kudriavzevii x S. cerevisiae yeast hybrids are frequently used to produce wines. The enological characterization of several of those S. cerevisiae x S. kudriavzevii hybrids have suggested that the S. cerevisiae genome contributes to ethanol tolerance and elevated fermentative capacity5,6, whereas the S. kudriavzevii genome provides adaptation to low temperatures7,8. S. kudriavzevii produce higher amounts of glycerol during wine fermentations3,5, a characteristic that has been linked to cell survival in fermentations at low temperatures9 and has also been found to be involved in freeze-thawing stress resistance10.
This work presents a transcriptomic study of S. cerevisiae x S. kudriavzevii hybrids, aimed at deciphering the molecular adaptation of these strains to low temperatures. To perform this study, we used two wine yeast strains of S. cerevisiae x S. kudriavzevii hybrids that are commercialized in the market as cryophilic strains, Lalvin W27 and VIN7. These strains were selected according to previously published data6,11 and showed differences in genomic parental allele composition. Although both of them are allotriploid hybrids, the chromosomes inherited from each parent are different. W27 has lost part of chromosome IV, IX and XV of the S. kudriavzevii genome6, whilst VIN7 has lost chromosomes III and parts of chromosomes IV and VII11,12.
The yeasts strains included in this study were the strain Lalvin T73 (S. cerevisiae) and the strain IFO1802 (S. kudriavzevii), used as the parental species, and two S. cerevisiae x S. kudriavzevii hybrids, VIN7 and Lalvin W27, isolated from wine in South Africa and Switzerland, respectively. The yeast was grown and maintained in GPY medium (2% glucose, 0.5% peptone, 0.5% yeast extract) and plates (with 2% agar). Wine fermentations were performed in grape juice from the Tempranillo variety at 28°C or 12°C in vessels with 0.45 L of Tempranillo grape must.
Each yeast strain had two independent fermentations and three samples were taken from each independent fermentation. Cells from each yeast strain fermentation were pelleted by centrifugation (4000 rpm/min, 5 min), at 12°C and 28°C. Cells were collected at the beginning of the exponential phase by taking samples two generations after inoculation. The RNA extraction protocol was based on subsequent treatments with phenol-tris, phenol-chloroform (5:1) and chloroform-isoamyl alcohol (24:1), and an ethanol precipitation with sodium acetate13. RNA concentrations and purity were determined using a Nanodrop spectrophotometer ND-1000 (Nanodrop Technologies™, Wilmington, DE). RNA integrity was checked by electrophoresis in agarose gel (1%). 2–4 μg of total RNA from each sample was linearly amplified using the Low RNA Input Fluorescent Linear Amplification kit (Agilent Technologies™, Ca, USA). 2–3 µg of amplified cRNA was used as template for cDNA synthesis. cDNA was marked indirectly using the SuperScript™ Indirect cDNA Labeling System (Invitrogen™, San Diego, CA). Cy3 and Cy5 mono-reactive Dye (Amersham GE Healthcare™, Amersham UK) were used as the fluorophores and dye incorporation was monitored using a Nanodrop spectrophotometer.
A 200 to 300 pmol mixture of the labelled cDNA samples was concentrated (Concentrator Plus, Eppendorf™, Hamburg, Germany). Competitive hybridization was performed on a Yeast 6.4K Array with PCR-amplified ORFs of yeast S288c strain (Microarray Centre, UHN, Toronto, Ontario, Canada) in AHC hybridization chambers (ArrayIt Corporation, CA, USA) at 42°C overnight. Heterologous conditions as stated by Gamero et al.14 were employed to assure the hybridization of the S. kudriavzevii genome. The pre-hybridization solution contained 3X SSC, 0.1% SDS and 0.1 mg/ml BSA; the hybridization solution contained 0.1% SDS, 0.1 mg/ml of salmon DNA and 5X SSC. Microarrays were manually washed with different solutions containing different SSC 20X and SDS 10% concentrations (Sol.1: 0.1% SDS-2X SSC; Sol.2: 0.1% SDS-0.1X SSC; Sol.3: 0.1 SSC; Sol4: 0.01X SSC). The signal intensities of Cy3 and Cy5 were acquired with an Axon GenePix 4100A scanner (Molecular Devices, CA, USA) using GenePix Pro v.6.1 software, at a resolution of 10 µm.
Microarray data were derived from three independent cDNA hybridization experiments. Background correction was performed with GenePix pro 6.0. Subsequent analyses were performed with the Acuity 4.0 software (Molecular Devices, CA, USA). Individual datasets were normalized to log2 ratio value of 1 and data were filtered to remove spots that were not flagged and manually processed for print tip effect corrections. Only spots data with a minimum of two replicates were considered. Replicates were combined and medians were calculated. The first cut-off was the selection of the genes presenting at least 2-fold log2 ratio values, according to the literature15–17. These genes underwent a “GO term” enrichment analysis using the GO Term Finder tool in the Saccharomyces Genome Database (http://www.yeastgenome.org/). Regarding the statistics, False Discovery Rate (FDR) analysis and a significance level of 99% (p value < 0.01) were applied.
Expression of genes NUG1, LSM8, PDC5, NSR1, GPD1 and GUT2 was investigated by qPCR. Normalization of gene expression was carried out using ACT1 and RDN18-1 as controls since their expression remains constant along fermentation. Primers were designed using Primer-BLAST (NCBI) and S. kudriavzevii and S. cerevisiae gene sequences were deposited in databases (www.ncbi.nlm.nih.gov; GSE90793). Forward and reverse oligonucleotides were synthetized to hybridize to the selected alleles of S. kudriavzevii or S. cerevisiae genes (Table 1). PCR Mastercycler pro (Eppendorf, Germany) confirmed the allele primer specificity and their annealing temperature. cDNA synthesis and RNA extraction were carried out as previously explained. qPCR runs were done in triplicate in a LightCycler® 480 Real-Time PCR System (Roche, Switzerland) and analyzed using the software from the manufacturer (LightCycler® Software, version 4.0). The relative gene expression was quantified by comparison with ACT1 and RDN18-1 expression, after confirm comparable PCR efficiency.
Yeast cells grown overnight in GPY were diluted to 2 × 106 cells/ml the next morning. They were then grown until the mid-log phase (approximately 1 × 107 cells/ml), and 175 μl were inoculated on each GPY plate. A filter (1 cm diameter) with paromomycin (2 μg) was placed on the surface and plates were incubated at 12°C or 28°C until the lawn was formed. The assays were repeated twice.
We carried out micro-fermentations in vessels with 0.45 L of Tempranillo grape must, mimicking previous work conditions4. The time needed for both hybrid strains to finish the fermentation at 28°C was similar, 5 and 6 days for Lalvin W27 and VIN7, respectively (Table 2). In contrast, at low temperature (12°C) the fermentation performance was quite different. W27 behaved more similar to the S. kudriavzevii type strain (11 days), taking 14 days to finish fermentation. Hybrid strain VIN7 performance was more similar to the S. cerevisiae parental strain (21 days) and took 23 days. The differences in fermentation kinetics amongst these strains revealed how allelic differences can determine the behaviour of oenological traits of interest.
To evaluate changes in the global expression of genes during acclimation to low temperature in the wine fermentation of natural must, sampling was done at the beginning of the exponential phase when cells are growing at a speed close to µmax, two generations after inoculation. RNA from the samples was hybridised against the S288c microarray to study transcriptomic changes. An average of 86% sequence similarity exists between species of S. cerevisiae and S. kudriavzevii4, so we used heterologous hybridisation conditions for the microarrays. We observed that 95% of the total S288c gene spots were hybridised by S. kudriavzevii DNA4. Gene expression of each strain at both temperatures was analysed. Genes that were differentially expressed at 12°C and 28°C at a level that was considered significant were analysed further using the SAM (Significance Analysis of Microarrays) test with an FDR below 5% (Supplementary Table 1).
In S. cerevisiae x S. kudriavzevii hybrid strain VIN7 at low temperature, 18 genes were up-regulated, while 22 genes were down-regulated. For W27, 20 genes were up-regulated while 3 genes were down-regulated. GO analysis using the GO Term Finder on the Saccharomyces Genome Database was performed to evaluate the GO categories arising from the up- and down-regulated genes in both hybrid strains (Supplementary Table 2). Analysing the up-regulated genes in both strains after applying the GO-module online tool (http://www.lussiergroup.org/GO-Module) for false positives resulted mainly in GO terms related to “magnesium ion binding”, “thiamine pyrophosphate binding”, “branched-chain-2-oxoacid decarboxylase activity” and “pyruvate decarboxylase activity”. The last category appeared, because amongst the up-regulated genes we found the three pyruvate decarboxylases PDC1, PDC5 and PDC6, together with IDP2. The electron transport category was also significantly up-regulated in W27, similar to what has been previously described in S. cerevisiae and S. kudriavzevii4. Amongst other up-regulated GO-terms in W27 is also “amino acid metabolism” with a number of sub-categories, and in “heavy metal binding” in VIN7. These GO-terms were also previously observed in S. kudriavzevii. For the hybrid strain VIN7 the genes down-regulated at 12°C were related to rRNA synthesis, while for W27 no GO term categories were found.
Overall, the most remarkable group of regulated genes (either up or down-regulated) in the hybrid strains fell into the translation machinery efficiency category, as previously shown for S. kudriavzevii compared to S. cerevisiae at 12°C4. When comparing the two strains that showed high fermentation performance at 12°C (S. kudriavzevii IFO1802 and the hybrid W27) to the two strains with low fermentation performance at 12°C (S. cerevisiae T73 and VIN7), the cold adapted strains overexpressed 13 genes, including NUG1, involved in rRNA export18, and chaperones DDR4819 and SRP120 that couple proteasomes to polypeptides emerging from the ribosome (Supplementary Table 1).
An open question regarding yeast hybrids is the relative contribution of the different parental alleles to the total expression of specific genes. To test the role of the different alleles (S. cerevisiae or S. kudriavzevii) in the better adaptation to cold temperatures, we selected three differentially overexpressed genes in W27 in this work, NUG1, PDC5 and LSM8, and also three S. kudriavzevii cold stress markers described in different studies, NSR1, GPD1 and GUT23,4,21. We observed overexpression of the overlapping dubious ORF YJR023C for LSM8 and assumed that this overexpression was effectively in LSM8. NSR1, LSM8 and NUG1 are related to translation machinery efficiency, whereas GPD1 and GUT2 are related to glycerol metabolism, involved in cold adaptation. GPD1 and GUT2 are also involved in NAD+/NADH balance, with PDC5. In Table 3 we included the genomic configuration, obtained from previous work6,11,12, for the selected genes in each of the two hybrid strains, showing that most genes have at least one copy of each parental allele with the exception of S. kudriavzevii allele losses for NSR1 in VIN7 and for GPD1 and GUT2 in the W27 strain. The results do not suggest a general correlation between the relative contribution of S. kudriavzevii alleles to total gene expression of key genes and the cryotolerance shown by W27.
Sc: S. cerevisiae; Sk: S. kudriavzevii. Sc/Sk is the relative expression of Sc alleles divided by relative expression of Sk. nd: not determined; <dl: below detection limit. C: S. cerevisiae allele; K: S. kudriavzevii allele. All values were normalized relative to the W27 LSM8 S. cerevisiae allele. *: Data obtained from previous work6,11,12.
W27 has one copy of the NSR1 S. cerevisiae allele, whereas VIN7 has three copies, but the S. cerevisiae allele expression is higher in the W27 strain. Thus, despite the higher number of total S. cerevisiae allele copies in VIN7, relative gene expression of the S. cerevisiae NSR1 allele is higher in W27, and also total relative expression is higher in W27. One explanation is that the S. kudriavzevii allele in W27 may promote expression of both alleles. NUG1 is another example of gene that does not show correlation between the number of copies and the level of expression. This gene has two copies of the S. kudriavzevii allele in W27 and one in VIN7 but shows higher expression in VIN7 than in W27. No expression was found for GUT2, but GPD1 expression was remarkably higher in the VIN7 strain mainly due to the high S. kudriavzevii allele contribution. There is also a negative correlation between the copy number of the S. cerevisiae allele for the PDC5 gene and gene expression. Despite the similar expression of the S. cerevisiae allele for the PDC5 gene between both strains, we observe that the Sc/Sk ratio is higher in VIN7 than W27, which suggests this gene may have impact on efficiency of cold vinifications. The Sc/Sk ratio is the relative expression of S. cerevisiae alleles divided by the relative expression of S. kudriavzevii alleles.
The expression of genes related to translation efficiency in the hybrid strains prompted us to study this phenotype. The translation efficiency of the hybrid strains compared to the parental strains at low temperature was analysed by testing their sensitivity to paromomycin, a potent inhibitor of translation22.
As it can be seen in Figure 1, the S. cerevisiae strain shows a growth inhibition halo at 12°C, whereas no inhibition halo can be seen in the S. kudriavzevii strain at 12°C, confirming that the translation efficiency is not compromised for this yeast at low temperatures. S. kudriavzevii showed growth defects at 28°C, whereas S. cerevisiae remained unaffected. The hybrid strains, on the other hand, clearly show growth defects at both temperatures. This means that 28°C is not an optimal growth temperature for either of these hybrids. An important difference between the hybrid strains is that W27 shows a narrower halo at 12°C compared to 28°C while for the VIN7 strain the situation is the opposite, supporting the idea of W27 being more similar to S. kudriavzevii strains in low temperature conditions.
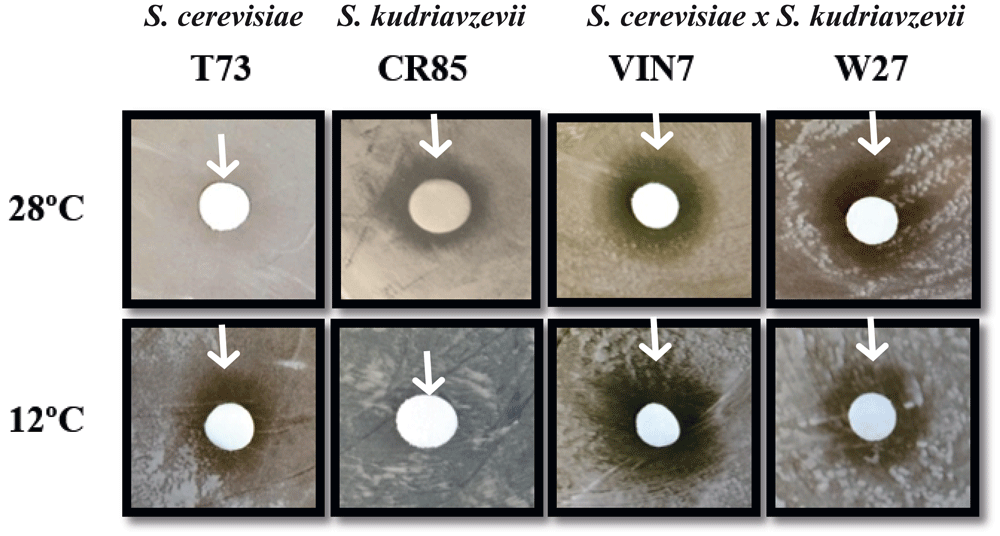
The inhibitory effect of the translation inhibitor paromomycin was evaluated by observing the halo diameter generated in the lawns of S. cerevisiae (T73), S. kudriavzevii (CR85) or S. cerevisiae x S. kudriavzevii hybrids (VIN7 and W27) growing in GPY plates at 28°C or 12°C. Representative experiments are shown, and the end of the inhibition halos are indicated with arrows.
In a previous paper, we analysed the transcriptomic behaviour of S. cerevisiae and S. kudriavzevii strains under low temperature fermentation conditions. Transcriptomic data showed the differences in gene expression between both species and also highlighted that the translation efficiency under low stress temperatures was higher in S. kudriavzevii than in S. cerevisiae4. In this work, we wanted to further investigate the transcriptomics of S. cerevisiae x S. kudriavzevii hybrids at low temperature and compare the translation efficiency to previous data on S. cerevisiae. The results show that hybrids maintain the winemaking abilities classically attributed to S. cerevisiae, with increased expression of fermentation related genes. This explains their advantage in the winemaking environment over other natural species like S. kudriavzevii, which cannot compete with S. cerevisiae even in low temperature conditions7. The hybrids also showed increased expression of genes related to cold adaptation, such as ribosome management genes (NUG1, SRP1), and also displayed paromomycin resistance, which confirmed this adaptation. Resistance to paromomycin is the result of enhanced translation efficiency due to an increased number of ribosomes available to a new round of mRNA translation. We cannot discard that differences in paromomycin resistance are due to mutations in genes unrelated to translation, as it has been described before23–26. However, this possibility is unlikely since enhanced resistance phenotype at low temperatures has been observed in two different S. kudriavzevii strains4 and, to less extent, in the two S. cerevisiae x S. kudriavzevii hybrids. In addition, our previous work have related paromomycin resistance with translation efficiency in these S. kudriavzevii strains by performing 35S-Methionine incorporation assay, showing that enhanced translation efficiency can be an adaptation to grow at low temperatures and alow adapted cell to shorten lag phase and resume earlier growth when cold is present4. In total, these results suggest that hybrids maintain both fermentative and cold adaptation abilities attributed to each parental species. This highlights their industrially relevant characteristics.
In our attempt to determine the relative contribution of S. cerevisiae and S. kudriavzevii alleles to the total expression of genes in the hybrids, our results showed that allele copy number did not correlate with allele gene expression in the hybrid strains. Although the genomic contribution of S. kudriavzevii provides improved fermentation at colder temperatures, the reason cannot be explained solely by the presence of these allele copy numbers. It must be taken into account that the S. kudriavzevii genomic contribution in these hybrids is smaller than the S. cerevisiae genomic contribution, and probably under the control of S. cerevisiae genomic regulators. The equilibrium acquired between the genomes of S. cerevisiae and S. kudriavzevii in stable hybrid strains is the result of a complex process aiming to improve environmental adaptation, and cannot be explained only by the sum of both genomes.
The microarray data discussed in this publication can be found in the Gene Expression Omnibus database (NCBI), with accession number GSE90793.
AQ and JMG conceived the study. AQ and JMG designed the experiments. JT and EGR carried out the research. JT and RPT prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
This work was supported by grants AGL2012-39937-C02-01 and AGL2015-67504-C3-1-R from the Spanish Government and ERDF (European Regional Development Fund) and by grant PROMETEO (project PROMETEOII/2014/042) from Generalitat Valenciana to AQ.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Supplementary Table 1. Transcriptomic dataset with genes that were differentially expressed at 12°C and 28°C genes in S. cerevisiae x S. kudriavzevii hybrids (VIN7 and W27) when compared to S. cerevisiae (T73) and S. kudriavzevii (CR85). The genes that were considered significant were analysed further using the SAM (Significance Analysis of Microarrays) test with an FDR below 5%.
Click here to access the data.
Supplementary Table 2. GO analysis of the differentially expressed genes at different temperatures in S. cerevisiae x S. kudriavzevii hybrids (VIN7 and W27). GO analysis using the GO Term Finder on the Saccharomyces Genome Database was performed to evaluate the GO categories arising from the up- and down-regulated genes in both hybrid strains. Analysis of the up-regulated genes in both strains was corrected for false positives after applying the GO-module online tool (http://www.lussiergroup.org/GO-Module).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast biology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Li M, Tzagoloff A, Underbrink-Lyon K, Martin NC: Identification of the paromomycin-resistance mutation in the 15 S rRNA gene of yeast mitochondria.J Biol Chem. 1982; 257 (10): 5921-8 PubMed AbstractCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 12 Sep 17 |
read | |
|
Version 2 (revision) 02 Jun 17 |
read | read |
|
Version 1 15 May 17 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)