Keywords
Complication, etofenamate, Nicolau syndrome
Complication, etofenamate, Nicolau syndrome
Nicolau syndrome is a rare complication caused by intramuscular injection of various medications1. The necrosis in the injection site of skin and sometimes muscle is a characteristic feature of this syndrome1. The development of acute vasospasm following intravenous or around the vein injection is the most widely accepted hypothesis in its pathogenesis1. Etofenamate is an anti-inflammatory drug that non-selectively inhibits the cyclooxygenase (COX) pathway2. Herein, we present a rare case of Nicolau syndrome after etofenamate injection.
An 81-year-old woman was admitted to our clinic with a painful necrotic ulcer in the left gluteal region. Her medical history, which was non-specific, except for back pain, revealed an intramuscular etofenamate injection (1000 mg), due to back pain, 15 days before. Dermatological examination revealed a painful ulcerous plaque with a black necrotic crest in the lateral part of the left gluteal region. This ulcerous plaque appeared indurated and erythematous in its surrounding (Figure 1). Her complaints started with erythematous swelling and pain in the injection site approximately ten days ago. Subsequently, the ulcer developed in the lesion area of the patient's erythematous swelling. There were not any abnormal parameters in both complete blood count and routine biochemistry tests. The patient was diagnosed with Nicolau syndrome based on her medical history and clinical signs and symptoms. Biopsy from the lesion area was not obtained, as it could develop more necrosis in the lesion. Etofenamate treatment was discontinued.
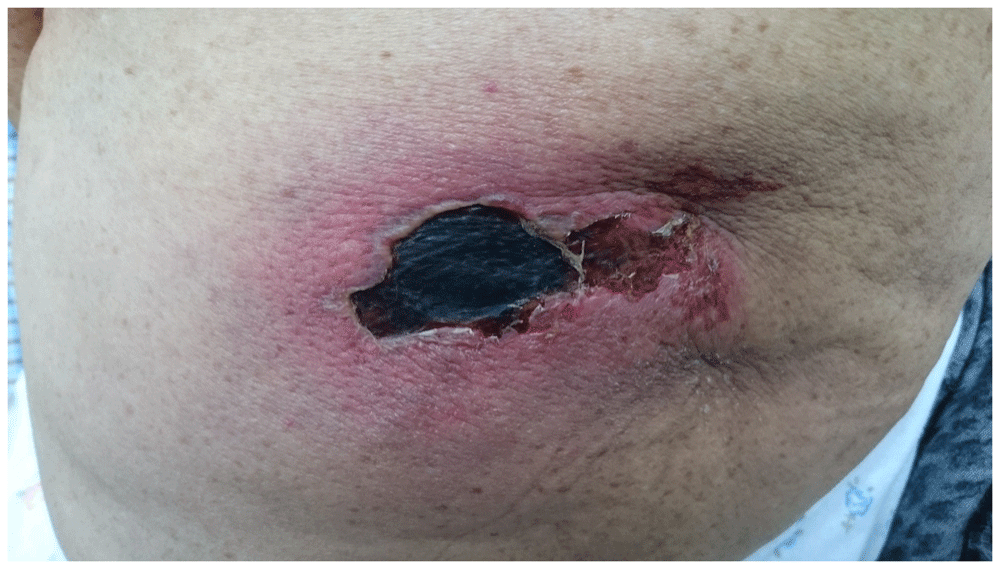
Local wound care with saline solution once a day and topical 2% mupirocin twice a day was applied to the lesion and the patient was referred to the Department of Plastic Surgery for the debridement of the necrotic tissue. After surgical debridement by the plastic surgeon, and continuation of local wound care (as above), the ulcer lesion was completely regressed, leaving an atrophic scar after one month (Figure 2).
Nicolau syndrome, also known as embolia cutis medicamentosa, is defined as an iatrogenic syndrome following intramuscular injections. However, cases with Nicolau syndrome after subcutaneous, intravenous, or intraarticular injection have been recently reported in the literature3–5.
Although the pathogenesis of Nicolau syndrome is not fully understood, direct vascular damage, perivascular inflammation, and vascular contraction following an injection are thought to be responsible6. In addition, it has been suggested that pharmacological properties of an individual drug may play a role in the pathogenesis6.
Etofenamate is a non-steroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic, and anti-inflammatory effects. It inhibits the COX pathway and blocks prostaglandin synthesis non-selectively2. It has been shown that NSAIDs play a key role in the pathogenesis of vascular spasm induction and local circulation blockage, inhibiting the COX enzyme and prostaglandin synthesis7. In addition, these drugs have a central role in inducing vascular spasm and blocking local circulation, inhibiting the COX enzyme and prostaglandin synthesis in the pathogenesis of this syndrome7.
In Nicolau syndrome, following the injection of the clinically active agent, erythematous, ecchymosed, and reticular lesions appear in the injection site with severe pain. Progressive ischemic necrosis with sharp edges in a livedoid pattern develops later. Lesions often heal leaving atrophic scars8.
Nicolau syndrome has no definitive treatment. In the early period, the main goal of therapy is to prevent the development of necrosis. Therefore, pentoxifylline, hyperbaric oxygen, intravenous alprostadil, and heparin, which strengthen the vasculature, can be used4. Intralesional steroid injection can also be effective by reducing inflammation. Surgical debridement should be performed in the case of necrosis4. Systemic antibiotics should be used in case of secondary infection4. Contraction and deformity development are among late complications, and surgical treatment can be required in these cases9. Nicolau syndrome is uncommon with proper injection techniques - aspirating just before injecting medication has been suggested as a technique of preventing this syndrome10.
As a result, applications of standard drug injection rules are essential in prevention from Nicolau syndrome. It should be kept in mind that Nicolau syndrome could also develop following the use of intramuscular etofenamate.
Written informed consent was obtained from the patient for the publication of the patient’s clinical details and accompanying images.
EO: wrote the manuscript; AB: Prepared the manuscript; RE, YU, KO and AA: Helped manage the patient’s diagnosis and therapy; HB: patient’s consultant from the Department of Plastic and Reconstructive Surgery
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 12 Jun 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)