Keywords
HIV-1, novel, correlates of protection, HIV-1 vaccines, HERV-K HML-2, HERV-K102, foamy macrophages, innate immunity
HIV-1, novel, correlates of protection, HIV-1 vaccines, HERV-K HML-2, HERV-K102, foamy macrophages, innate immunity
It has been recently stated that immune correlates of risk/protection for HIV-1 vaccines must be complex and/or reliant on the right combination of multiple types of immune responses, as correlates of protection have eluded investigators (Tomaras & Plotkin, 2017). However, the relatively low rates of acquisition of HIV-1 per exposure at less than 1 in 1000 for heterosexual transmission (Becerra et al., 2016), may instead argue that defense against HIV-1 in humans possibly involves a simpler and more potent mechanism than what has yet been elucidated or appreciated. Indeed, that a new scientific paradigm may be needed to advance the development of the HIV-1 vaccine has been recently proclaimed (Esparza, 2015). What then can we currently deduce about the characteristics of this unknown correlate of risk/protection, such as from the two informative HIV-1 preventative vaccine trials: one associated with increased HIV-1 transmission, known as the STEP trial (Buchbinder et al., 2008), while the RV144 trial that was associated with decreased HIV-1 transmission (Rerks-Ngarm et al., 2009)?
In any immune response, innate and/or adaptive, activated macrophages control the response. Accordingly, it follows that any risk or protection associated with HIV-1 vaccines must then relate to a key and so far, ill-defined macrophage activation pathway. Moreover, in HIV-1 acquisition, the transmitting/founder strains are generally CCR5-tropic and target macrophages (reviewed in Borggren & Jansson, 2015). Together these findings point to the likelihood that there exists a novel macrophage-based defense mechanism that itself determines whether HIV-1 will be acquired.
The Ad5 vector used in the STEP trial, highly targets liver Kupffer cells, which represent about 80–90% of the macrophages in the body (Khare et al., 2011). In the STEP trial, male uncircumcised participants with Ad5 antibodies were significantly at increased risk of transmission compared with those without vector antibodies (Buchbinder et al., 2008). This suggests that at the interface of the Ad5 vector with macrophages, the presence of bound Ad5 antibodies enriched in the local milieu may have somehow blocked the induction of the putative, gatekeeper/defense mechanism of macrophages. A possible candidate mechanism for this interference by antibody is tuftsin, a short peptide consisting of the sequence TKPR, which is released from bound IgG and is known to inhibit macrophage activation at higher concentrations, while at lower levels, it augments macrophage activation (Siemion & Kluczyk, 1999). Thus, it appears to be plausible that higher risk participants (uncircumcised men) with pre-existing Ad5 antibodies injected with an Ad5 vector could have experienced inhibition of the putative, novel macrophage defense mechanism such as by the local generation of tuftsin, thereby explaining their increased risk.
Another important clue relates to gender. In the STEP trial, women were notably at decreased risk of HIV-1 acquisition when compared with males (Buchbinder et al., 2008), whereas in the RV144 trial protection against HIV-1 acquisition was more evident in females than males (about 1.5-fold better) (Rerks-Ngarm et al., 2009). Together these findings suggest the novel, macrophage based protection mechanism is likely induced by female hormones.
That the risk/protection was only temporary in either trial, generally lasting 6 to 12 months after the last immunization (Buchbinder et al., 2008; Rerks-Ngarm et al., 2009), was consistent with an innate rather than adaptive immunity mechanism. In addition, following HIV-1 acquisition, the levels of CD8 and CD4 positive, CD38 positive, DR positive activated T cells are well recognized progression markers (Deeks et al., 2004), indicating adaptive immunity is unlikely to provide significant protection against HIV-1 replication. Accordingly, the key protection mechanism must be innate, but this does not necessarily rule out important contributions of intrinsic factors, such as APOBEC, which may antagonize HIV-1 replication and may also be upregulated with alternative activation of macrophages (Colomer-Lluch et al., 2016; Hartmann, 2017).
Since both risk (Buchbinder et al., 2008) and protection (Rerks-Ngarm et al., 2009) were found with viral vectored vaccines, and not in the VAX003 and VAX004 HIV-1 vaccine trials, which instead involved proteins and adjuvants (reviewed in Shin, 2016), this raises the likelihood that viruses in general may activate the undefined macrophage-based defense mechanism, in addition to HIV-1.
Overall, these observations point to the existence of a novel, potent innate HIV-1 protection mechanism induced by viruses and female hormones, which is launched by activated macrophages, possibly using alternative pathways, and that this activation may be sensitive to inhibition by locally bound antibodies, for example by tuftsin. Moreover, despite a considerable effort both inside and outside of the trials, convincing immune correlates of risk or prevention against HIV-1 have yet to be revealed. This failure raises the following notions about the mechanism. It is likely not addressed by traditional in vitro culture methods, not discoverable using conventional detection methods on participant samples, including microarray and genome wide association studies, nor is it likely present in the rhesus macaque. The critical question becomes, has such a novel, innate, potent, defense mechanism unique to human activated macrophages and difficult to study under standard conditions, been previously described?
Surprisingly, the answer to this may be, yes.
A novel, innate, viral defense mechanism unique to humans, associated with the production of foamy macrophages in vitro (Figure 1) was serendipitously discovered by scientists working at the Public Health Agency of Canada about 10 years ago, and fulfills all the above criteria for a defense mechanism launched by alternatively activated macrophages (Laderoute et al., 2007; Laderoute et al., 2015; Laderoute, 2015).
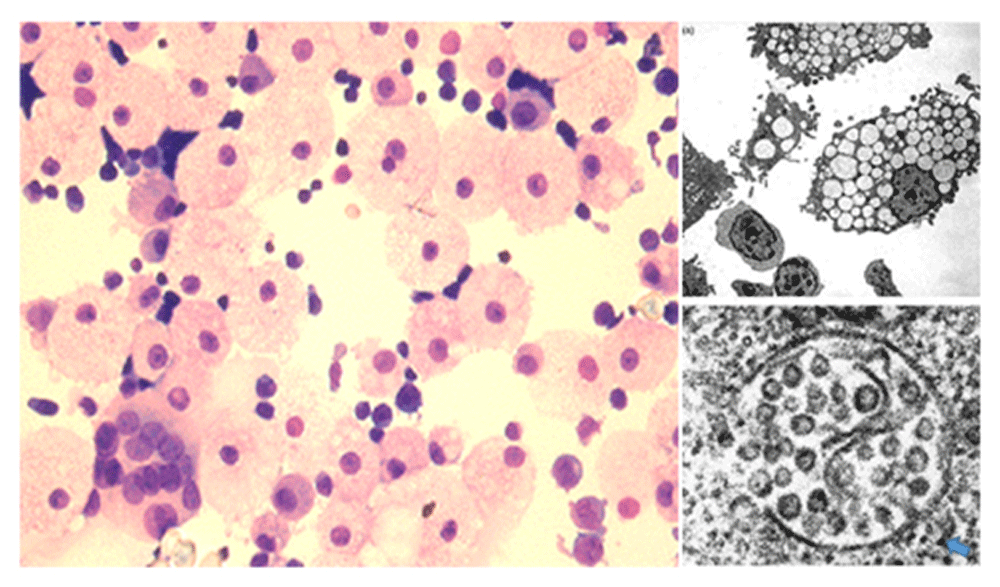
Left panel, H and E stain of day 11 cultured CB prepared by cytospin. Right panels, electron micrographs show vacuoles in the foamy macrophages contain large numbers of immature particles with envelope spikes. Blue arrow points to the cytoplasmic capsid assembly outside of the vacuole, typical of foamy retroviruses (Hütter et al., 2013). Left panel reproduced under a CC BY- NC 4.0 license (Laderoute et al., 2015). Right panels reproduced with permission from the AIDS journal (Laderoute et al., 2007).
Importantly, this also includes initial evidence that it was associated with protection against HIV-1 acquisition in a female, HIV-1 highly exposed seronegative cohort (HESN), at the level of about 80% of the tested cohort (Laderoute et al., 2015). Moreover, protection in the infamous Nairobi HESN cohort (Fowke et al., 1996) is known to be temporary, as resistance to HIV-1 acquisition dwindled as early as 6 months to a year following abstinence from sex trade work (Kaul et al., 2001). Thus, this novel foamy macrophage mechanism, which might be implicated in the Nairobi HESN cohort, seems to meet a key criterion of temporary activity and waning after 6 months.
This putative defense mechanism launched by activated foamy macrophages in vitro (Figure 1) was characterized as human endogenous retrovirus K102 (HERV-K102, GenBank accession # AF164610) (Laderoute et al., 2015). HERV-K102 is an endogenous retrovirus unique to humans (Subramanian et al., 2011), and is the only HERV-K HML-2 group member which has been shown to be replication competent in vivo and as associated with viremia (Laderoute et al., 2007). As extensively reviewed elsewhere (Laderoute et al., 2015), it has hallmark features of non-pathogenic, foamy retroviruses. Like foamy viruses, its genomes are predominantly DNA rather than RNA (Laderoute et al., 2007) and replication involves budding into vacuoles rather than the cell surface, which renders cells foamy (Figure 1). It is important to note that the particles are released by cell lysis of the foamy macrophages and that foamy macrophages do not express HERV-K102 envelope at the cell surface (unpublished studies; Marian Laderoute). When cord blood mononuclear cells (CB) were cultured in IMDM rather than RPMI media, HERV-K102 spontaneously replicated, generating high levels of foamy macrophages (Laderoute et al., 2007; Laderoute et al., 2015). Others have similarly reported the induction of foamy macrophages when CB was cultured in IMDM (Stec et al., 2007). HERV-K102 appears to be the only HERV-K HML-2 element so far shown to be naturally replication competent in vitro (Laderoute et al., 2015) and in vivo (Laderoute et al., 2007).
HERV-K102 particles released by freeze-thaw cycles of cultured CB cells, induced rapid and complete cell lysis of MRC-5 cells at 24 hours, which was not demonstrated for other cell lines tested (unpublished study; Marian Laderoute). This was expected as foamy viruses are well known to produce rapid cell lysis of some, but interestingly, not all fibroblastic cell lines (Linial, 2001). However, it remains to be determined which cell death pathway (Duprez et al., 2009) might mediate this remarkably rapid cell lysis, if integration is needed, and more critically, if HERV-K102 particles might similarly lyse HIV-1 infected cells. It is notable that HERV-K102 also appeared to be strongly induced in vivo increasing from no particles detected to 2.55 × 1011 cDNA containing particles per ml of plasma within 84 hours (unpublished study; Marian Laderoute). High levels of particles at 1010 to 1012 per ml of plasma were frequently found in patients viremic for various bloodborne pathogens, but maximum levels in HIV-1 patients were notably 7 to 8 log-fold downmodulated in comparison (Laderoute et al., 2007). It can be estimated from previously reported data that in HIV-1 patients there may only be on average about 8,200 DNA containing particles per ml of plasma corresponding to HERV-K102 (Laderoute et al., 2007), which has been substantiated by others but as demonstrated by the detection of excess HERV-K HML-2 transmembrane envelope DNA but not RNA sequences on isolated particles as compared with normal healthy controls. (Bhardwaj et al., 2014). It should also be noted that two groups have reported that HERV-K HML-2 particles with RNA genomes were not demonstrable in HIV-1 patients (Bhardwaj et al., 2014; Karamitros et al., 2016), consistent with the notion that HERV-K102 transcripts in particles are predominantly or exclusively DNA (Laderoute et al., 2007). Taken together these results may suggest only HERV-K102 particles with DNA genomes are significantly produced in HIV-1 patients, but this needs to be more carefully addressed. Thus, overall, based on rapid induction, the high levels of particles that can be produced both in vitro (Figure 1) and in vivo (Laderoute et al., 2007), and potentially swift cell kill by particles (unpublished study; Marian Laderoute), HERV-K102 particles could comprise a potent innate immune mechanism launched by foamy macrophages. However, upon HIV-1 acquisition, HERV-K102 particle production and/or release appear to be strongly inhibited.
In vitro, and despite suboptimal conditions by culture of the cells in RPMI, several research groups have confirmed that HERV-K102 is induced by HIV-1 (Brinzevich et al., 2014; Vincendeau et al., 2015). Moreover, HERV-K102 may be the only human specific, full length HML-2 element induced by HIV-1 and/or Tat (Gonzalez-Hernandez et al., 2012). While the envelope of HERV-K18 and a consensus sequence for HERV-K HML-2 were able to pseudotype HIV-1 virions, interestingly HERV-K102 did not (Brinzevich et al., 2014; Lee & Bieniasz, 2007). That HIV-1 may be pseudotyped by HML-2 envelope raises the notion that such pseudotyped particles could help explain, in part, the altered tropism for macrophages bearing the CCR5 coreceptor, which is commonly used by transmitting/founder strains (Borggren & Jansson, 2015). If this is indeed the case, it would also help explain why vaccination against HIV-1 envelope generally fails to prevent HIV-1 acquisition (reviewed in Shin, 2016), or why passive immunization with HIV-1 envelope specific, broadly neutralizing envelope antibodies failed to significantly control viremia upon antiretroviral treatment interruption (Bar et al., 2016; Caskey et al., 2017).
The mean HERV-K102 pol copy number in the genomes of the HESN, as demonstrated on DNA extracted from plasma, was elevated about 5-fold above the genomic levels of normal healthy adults (p<0.0005) (Laderoute et al., 2015). This is consistent with high integration levels reported for foamy viruses in hematopoietic cell lines (Meiering et al., 2000). This implied very high HERV-K102 particle production likely occurred in the HESN cases. In direct contrast, there was no evidence for increased mean genomic copy number above normal healthy controls for North American individuals already infected with HIV-1, irrespective of their use of anti-retroviral therapy (Laderoute et al., 2015). However, evidence of the activation of this macrophage-based defense system was demonstrated in about 96% of HIV-1 patients which includes particles and/or HERV-K102 surface unit envelope specific antibodies (post hoc analysis, Laderoute et al., 2007). Thus, as might be expected, individuals protected against HIV-1 acquisition may produce high numbers of HERV-K102 particles reflected by increased integration, but upon its acquisition, HERV-K102 particle production was strongly downmodulated with no evidence of increased integration of HERV-K102 sequences (Laderoute et al., 2007; Laderoute et al., 2015).
In potential substantiation of an important role of HERV-K102 in the control of HIV-1 replication, HERV-K HML-2 gag and envelope RNA expression in peripheral blood mononuclear cells (PBMCs) in HIV-1 patients were shown to be inversely correlated with T cell activation markers (Ormsby et al., 2012). Since it is known that activated T cells correlate with HIV-1 progression (Deeks et al., 2004), this implies HML-2 expression generally, and by proxy HERV-K102 activation, may antagonize HIV-1 replication. This argument is further strengthened by recent evidence that suggests the newer HERV-K HML-2 elements containing LTR5Hs (which include HERV-K102) are upregulated in CD11c+ myeloid dendritic cells isolated from HIV-1 patients, whereas, in normal healthy controls, the older LTR5A and LTR5B bearing HML-2 elements prevailed (Young et al., 2014).
Both antibodies and T cell responses to HERV-K HML-2 and/or HERV-K102 envelope have been demonstrated in HIV-1 and breast cancer patients (reviewed in Laderoute et al., 2015). A T cell clone isolated from an elite controller, which recognized a peptide identical to HERV-K102 envelope, was shown in vitro to specifically eliminate cells infected with various HIV and SIV strains (Jones et al., 2012). Remarkably, a monoclonal antibody made to HERV-K102 envelope could directly provoke apoptosis in vitro and in vivo (Wang-Johanning et al., 2012). This might suggest that the expression of HML-2 envelope on the surface of virally infected or transformed cells, but which is not found on normal cells, plays a more active role in innate host protection than merely as a surrogate marker. These findings may also further document the unexpected potency of this innate protector mechanism against HIV-1, which unlike adaptive immunity, remarkably functions irrespective of the hypervariability of HIV-1, quasi-species and/or strains of HIV-1 or lentivirus involved.
In terms of other characteristics of the defense mechanism deduced earlier from the informative HIV-1 vaccine clinical trials, combination female steroid hormones (estrogen then progesterone) have been shown to stimulate the expression of HERV-K HML-2 (Ono et al., 1987). In a recent meta-analysis, while the use of various progestins for oral contraception were associated with a significantly increased adjusted hazard ratio of HIV-1 acquisition over women who did not use contraceptives, the combined oral contraceptive was not (Morrison et al., 2015). This is consistent with the notion that women of child-bearing age (and not on progestins), may be more protected against HIV-1 acquisition compared with male counterparts possibly through regular, monthly induction of the HERV-K102 protector system by combined estrogen and progesterone.
The identification and elucidation of correlates of protection against HIV-1 have been challenging. Overall the failure to identify HERV-K102 particles pertains largely to the notion that its presence is, more often than not, overlooked or not addressed by standard methodological approaches. For example, because HERV-K102 is unique to humans (Subramanian et al., 2011), it is absent from animal models, such as macaques and rodents, which are commonly used for vaccine or immunological investigations. In addition to HERV-K102 and HML-2 being inhibited when PBMCs or CBs are cultured in the more traditional RPMI media invariably used by immunologists (Argaw-Denboba et al., 2017; Laderoute et al., 2015), HERV-K102 activation is also blocked by the depletion of CD14 + cells from PBMC, and also by the addition of PHA and IL-2 to cultures performed in IMDM (unpublished studies; Marian Laderoute). Accordingly, it may not be a co-incidence that the conditions that block HERV-K102 particle production in vitro are those that instead are commonly employed to demonstrate HIV-1 infectivity, such as purified T cells activated with PHA and IL-2 cultured in RPMI. Indeed, these observations would be consistent with the possibility that HERV-K102 particles may antagonize HIV-1 replication in vitro; however, importantly this needs to be directly examined.
The detection of the presence of HERV-K102 particles also eludes other common approaches utilized for investigations. For example, detection of particles in plasma requires an alternative isolation strategy seldom employed by retrovirologists. It requires DNA and not RNA isolation from plasma (Laderoute et al., 2007), where the use of DNAse would be contraindicated. As well, genome wide association studies and microarray analysis typically exclude highly repetitious sequences (Baranzini et al., 2010; Held et al., 2003, respectively) to which this element belongs. Accordingly, HERV-K102 particle production appears to have eluded the field due to the difficulty in demonstrating its presence using standard or traditional approaches.
Relevant to the increased risk of HIV-1 acquisition related to Ad5 antibodies in the STEP trial (Buchbinder et al., 2008), at a high concentration (2 mg/ml), tuftsin inhibited the production of HERV-K102 DNA in cultured CB by 53%, while at a lower concentration (200 ng/ml), tuftsin enhanced the replication of HERV-K102 pol containing DNA over normal genomic levels by 237% (unpublished study; Marian Laderoute). Thus, it seems as a protector mechanism launched by alternatively activated macrophages, HERV-K102 particle production might be subject to modulation by tuftsin and thus possibly relevant to the adverse outcomes of the STEP trial. Clearly, further investigation of the mechanisms of how pre-existing antibodies were associated with adverse outcomes in the STEP trial appears to be warranted.
Accumulating phylogenetic evidence is consistent with a potential role of HERV-K HML-2 in limiting invasion by orthoretroviruses (Magiorkinis et al., 2015). Ancestral HML-2 elements emerged about 10.3 million years ago (Mya) (Subramanian et al., 2011). There has been a striking decline of insertions of ERVs in the last 10 My in the genomes of all sequenced hominids (great apes and gibbons), but not in old world monkeys (baboons and macaques), particularly regarding HERV-H (Magiorkinis et al., 2015). HERV-H makes up 88% of all the ERV integrations into the human genome in the last 30 My and became extinct over the past 10 My. HERV-H is a gammaretrovirus, which integrated around 45 to 60 Mya and has about 962 copies in the human genome (Chuong et al., 2016). HERV-K, with 10 groups in the clade, only one of which is HML-2, on the other hand, entered the genome of ancestral catarrhines about 32 to 44 Mya, after the split from New World monkeys and before the split of hominids from the Old World monkeys (Kim & Han, 2015). The sister lineages of HERV-K in most other catarrhines appear to have become extinct. Most remarkably, the HERV-K HML-2 group in humans is the only HERV-K that has continued to replicate since the origin of the catarrhines (Magiorkinis et al., 2015).
Accordingly, since phylogenetic evidence supports an association of HERV-K HML-2 activity with protection against integration of orthoretroviruses, this may help substantiate the claim that modern day HERV-K102 particles, along with expression of proteins from other HML-2 elements, might antagonize HIV-1 replication and/or prevent its acquisition.
Somewhat ironically, humans apparently acquired the HERV-K102 defense mechanism possibly between 500,000 and up to 2 Mya (Romano et al., 2006; Subramanian et al., 2011), from the same source of the modern HIV-1 pandemic strain; namely, chimpanzees.
The Homo-Pan split has been estimated at 6.6 Mya (Magiorkinis et al., 2015) or earlier at 7-8 Mya (Langergraber et al., 2012). As mentioned, the HERV-K HML-2 elements originated in primates about 10.3 Mya and the CERV-K102 sequence (DQ112149), which is 97% identical to HERV-K102, was estimated to have integrated into chimpanzees at a non-orthologous position about 10 (+/- 3.3) Mya (Romano et al., 2006). Lentiviruses may have been active in primates since the divergence of chimpanzees and humans (Katzourakis et al., 2007; Sawyer et al., 2004). Moreover, it has been suggested the ancestor to HIV-1 may have arisen in chimpanzees about 4 Mya (Gifford, 2012). Since, it has been reported that subsets of chimpanzees with chronic HIV-1 infection showed progression analogous to humans, including greater expression of CD38 in CD8+ HLA-DR+ T cells (O’Neil et al., 2000), this raises the notion that an HERV-K102 ancestor, as a potential antidote for HIV-1 infection may have been selected through evolution in chimpanzees before it was acquired by humans. Accordingly, it is possible over about a 2 to 3.5 million-year window or longer, the HERV-K102 ancestor may have adapted to an HIV-1 like ancestor lentiviruses in chimpanzees prior to its acquisition by humans.
This inquiry has led to the notion that HERV-K102 particle production, which generates foamy macrophages, appears to fulfil the requirements of a deduced candidate correlate of protection against HIV-1 acquisition. Moreover, this candidacy has been strengthened by biological, clinical and phylogenetic evidence, including that which implies HERV-K102 particles may be associated with protection against HIV-1 acquisition. That conversely, acquisition of HIV-1 would be associated with significantly log-lower levels of HERV-K102 particles, would be anticipated and was observed. Given also the preliminary evidence that tuftsin could block the replication of HERV-K102 in vitro, suggests the blocking of the same mechanism, such as by Ad5 antibodies in the STEP trial shown in males at higher risk, could plausibly account for the increased risk observed in this informative trial. Finally, that the host source of this remarkable innate protection mechanism appears to be the same as that for pandemic strains of HIV-1 would strengthen its authenticity, especially given the likelihood of millions of years of co-evolution of HERV-K102 and HIV-1 in chimpanzees. Overall, the available evidence substantiates that a special antagonistic relationship exists between HIV-1 and a foamy-like virus, HERV-K102.
Accordingly, it will be extremely important to prioritize the testing of human endogenous retrovirus K102 (HERV-K102) particle production, integration, and/or envelope specific antibody production to prove or disprove it as a correlate of risk/protection on actual STEP and RV144 clinical trial participants (Figure 2). Exploratory studies in other HESN cohorts and in elite controllers may also serve to further strengthen the correlation. No less significantly, the clinical ramifications of pseudotyping of HIV-1 virions by HML-2 envelope needs to be addressed as it may also help explain in part the failed vaccine and cure attempts.
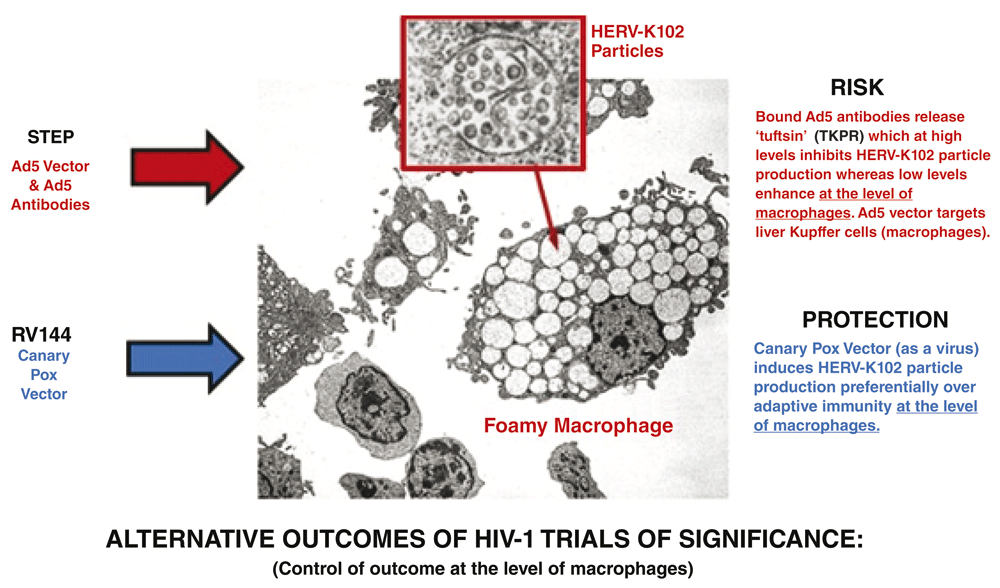
Adapted from Figure 1. Note that in the STEP trial, in addition to Ad5 antibodies, Th1 cytokines, such as might be released by Ad5 or HIV-1 antigen responsive T cells, could potentially also block HERV-K102 particle production in macrophages since PHA with IL-2 blocked HERV-K102 particle production in vitro (unpublished study; Marian Laderoute). Consistent with this notion, lower serum levels of IL-2, IFN-γ, and GM-CSF were recently demonstrated in a cohort of HESN over HIV-1 unexposed controls (Jaumdally et al., 2017), and IFN-γ levels were higher in HIV-1 positive over HIV-1 negative individuals (Yong et al., 2016).
No competing interests were disclosed. While the author was named as one of the inventors in patent applications for the discovery of HERV-K102 as a replication competent foamy-like virus and for envelope specific antibodies either of which may have applications in the field of infectious diseases, by policy at the Public Health Agency of Canada, public servants have no rights nor entitlements.
The author declares that no grants were involved in supporting this work. However, unpublished studies cited were previously supported by funding from the Blood Safety Program at the Public Health Agency of Canada and in part by an Innovative Research grant from the Office of the Chief Scientist at Health Canada.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
No
Are arguments sufficiently supported by evidence from the published literature?
No
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vaccines, HIV epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 25 Jan 18 |
read | read |
|
Version 1 12 Jun 17 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)