Keywords
cervical cancer, HPV, HPV integration sites, microRNAs, miRNAs, secondary structure, human genome variants, bioinformatics tools
cervical cancer, HPV, HPV integration sites, microRNAs, miRNAs, secondary structure, human genome variants, bioinformatics tools
Cervical cancer (CC) is the second most common malignancy in women worldwide. According to GLOBOCAN reports, approximately 530,000 women are diagnosed with CC and 265,672 die from it each year1. Infection by human papillomavirus (HPV) has been recognized as the major risk factor in this pathology2,3, but the virus presence is not the main cause for the development of this cancer4,5. Viral DNA integration into the host cell genome is considered a conducive factor for cervical intraepithelial neoplasia (CIN) to develop into CC5–7.
Numerous microRNAs (miRNAs) have been identified in proximity to HPV integration sites8,9. miRNAs are a class of small (18 to 26 nucleotides length), noncoding, evolutionarily conserved RNAs that are processed from longer transcripts known as pre-miRNAs (60 to 100 nucleotides in length)10. They are located on regions known as fragile sites and distributed in intergenic, intronic and exonic segments of the human genome involved in cancer11,12. Functionally, they regulate post-transcriptional expression levels of up to 60% of total protein-encoding genes by binding their seed sequences (2–8 nucleotides length). The 5'-UTR end of the miRNA seed sequence is complementary to the 3'-UTR end of the target mRNAs13. This recognition event can affect the expression of important regulatory genes. Deregulation of genes such as tumour suppressor genes and oncogenes can lead to cancer development, including CC14–16.
Human genome variants generate different patterns of miRNA deregulation17, which can contribute to cancer development susceptibility, treatment efficacy and patient prognosis18–20. 99% of the human genome is genetically identical, and the remaining 1% is responsible for all human diversity. miRNAs represent a major part of this genetic variation21. miRSNPs (single nucleotide polymorphisms in miRNAs) are human polymorphisms at or near predicted miRNA target sites22. The occurrence of miRSNPs can influence miRNA functionality on all levels, including transcription, maturation, and mRNA target binding.
Knowledge on miRNAs related to CC development in human genome variants from Latin American populations is scarce. Thus, in this study, we mapped miRNAs associated with CC in human genome variants obtained from Colombia, Mexico, Peru and Puerto Rico. Complete genomes were included in this study. Additionally, the relationships between HPV integration sites, genes close to these sites, mapping profiles and mutation patterns for each of the miRNAs were estimated for each of the genome sequences. The objective of this research was to analyse how genetic variation of CC-associated miRNAs identified in previously reported HPV integration sites affects cell cycle regulatory genes in human genomic variants from Latin America.
Two hundred and seventy-two miRNAs associated with CC were selected as described in the systematic review published by Guerrero & Guerrero23. With the information contained in miRBase24–26, miRNAMap27 and miRNAstart, features, such as length, chromosomal and genomic location of pre-miRNAs and mature miRNAs, were analysed. The mature miRNA reference sequences were obtained in FASTA format from the miRBase database (Dataset 128).
Four human genome sequences were obtained from randomly selected female participants in the 1000 Genomes Project from Latin American populations22,29. Their codes were CLM (from Medellin in Colombia), MXL (from Los Angeles and of Mexican ancestry in the USA), PEL (from Lima in Peru) and PUR (from Puerto Rico). The control sequence was a variant that is phylogenetically distant to Latin American variants and identified with the code BEB (from Bangladesh and of Bengali ancestry). Access codes were obtained from the 1000 Genomes Project resources21,30. This information is summarized in Table 1.
Viral insertion sites and nearby genes on the human genome were identified with the UCSC Genome Bioinformatics search engine31,32. To establish possible functional relationships with the development of CC, functional information on the associated functions of these human genes was obtained from UniProt33,34.
According to Xia et al.35, the mature miRNA sequences are located in regions with pre-miRNA secondary structure complementarity (3' and 5'). In total, 445 miRNA sequences were analysed. The Blast-Like Alignment Tool (BLAT) available on the UCSC Genome Bioinformatics website was used for mapping the miRNAs associated with the full human genome with the following parameters: (a) genome, human; (b) assembly, Dec. 2013 (GRCh38/hg38); (c) query type, DNA; (d) sort output, query; and (e) score and output, hyperlinks. A matrix of chromosomal location data was built with Microsoft Excel 2013 (‘Matrix of data’ in Dataset 236). From this matrix, the miRNAs over HPV integration sites were manually identified.
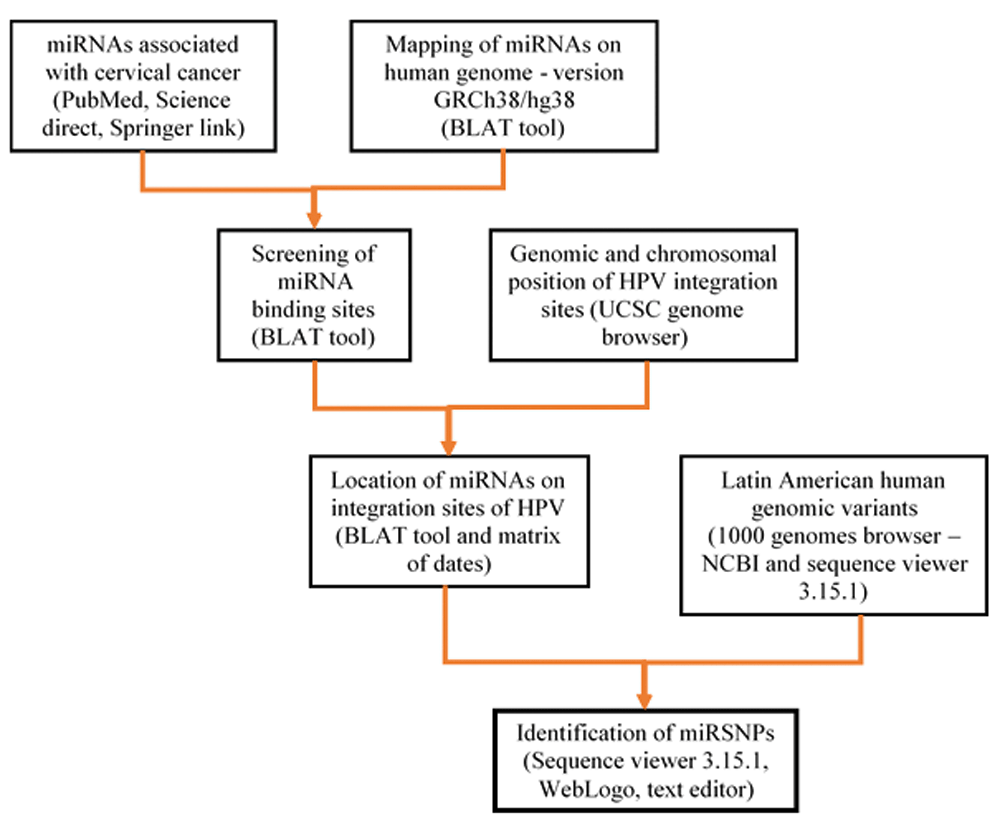
To identify miRNA mutations in the four Latin American human genome variants, the available tools, including ideogram view, subjects and exon navigator, in the NCBI 1000 Genomes Browser (Phase 3, version 3.7) were used. The code for each female genetic variant selection (Colombia, Mexico, Peru, Puerto Rico and Bangladesh) was inserted and the sequence of each miRNA identified in viral integration sites was introduced and the mapped nucleotide positions were selected. Using WebLogo 337, logos were created to view the nucleotide differences. The bioinformatics workflow is summarized in Figure 1.
A total of 44 publications were identified between 1987 and 2015 related to HPV integration sites in the human genome. The most frequent types of HPV associated with CC were HPV-16 and HPV-18. Details of these articles are outlined in Supplementary File 1. Five hundred and seventy-eight integration sites for 8 types of HPV associated with different histological cervical conditions were identified, of which 63.84% were HPV-16 (Figure 2 and ‘HPV integration sites’ in Dataset 236).
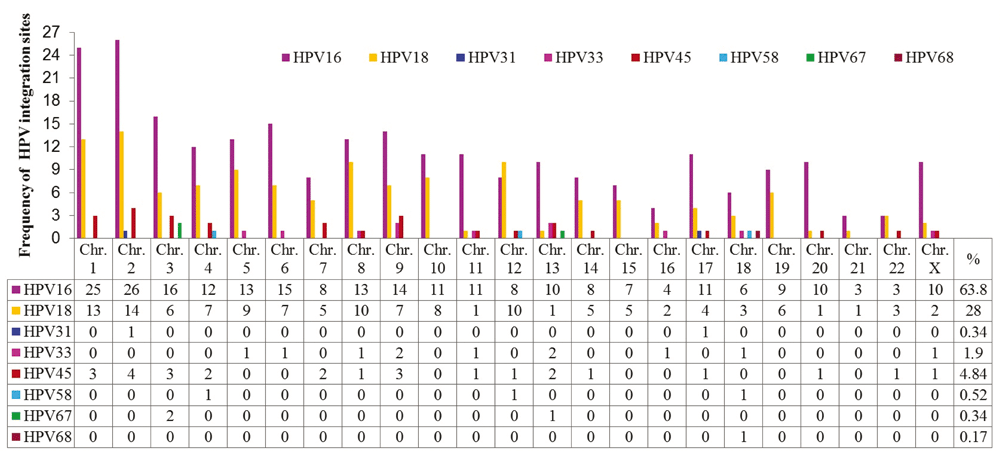
HPV-16 and HPV-18 have integration sites on all human chromosomes. HPV-16 has more integration sites on chromosomes 2, 1, 3, 6, 9, 5, 8 and 4, while HPV-18 has more on chromosomes 2, 1, 8, 12, 5, 10, 4, 6 and 9. Some less frequently oncogenic HPV types have integration sites on specific chromosomes, such as HPV-45 on 2, 1, 3, 9, 4, 7 and 13; HPV-33 on 9, 13, 5, 6, 8, 11, 16, 18 and X; HPV-58 on 4, 12 and 18; HPV-31 on 2 and 17; HPV-67 on 4 and 13; and HPV-68 on chromosome 18. Chromosomes 1 and 2 displayed a higher number of viral insertion sites (41 and 45, respectively), while chromosomes 13 and 18 displayed insertion sites for 5 different HPV genotypes. The chromosomal loci with the highest numbers of HPV integration sites are presented in Table 2.
Information on the associated functions of genes located near HPV integration sites obtained from UniProt showed that 86.1% of the genes located in close proximity were involved in apoptosis, cell adhesion, cell differentiation, ion transport and metabolic processes. Fifty-four genes were involved in direct regulation of the cell cycle. Twenty-six of these were tumour suppressor genes, 8 were oncogenes, 8 were proto-oncogenes and 12 did not have a determined functionality in the development of this neoplasia (Figure 3).
The 2028 miRNA binding sites associated with CC in the human genome were identified from BLAT mapping using previously identified miRNAs23, including 432 sites previously reported in miRBase (‘Results of mapping with BLAT’ in Dataset 236). These sites were located on both DNA strands (52.97% on the positive strand and 47.03% on the negative strand). 1881 binding sites were fully complementary (100% sequence identity) to miRNA sequences, while 1, 24, and 122 binding sites had 96.2%, 95.7% and 95.5% sequence identity, respectively.
miR-5095 was mapped onto 853 binding sites on 23 chromosomes. Four hundred and twenty-four mature miRNAs sequences (98.15%) mapped to one, two, three and even ten different binding sites. miR-522-5p and miR-523-5p binding sites mapped only a single chromosome (Chr. 19). Table 3 shows the chromosomal location and number of binding sites for each specific miRNA associated with CC.
The distribution of the 2028 binding sites was not homogeneous along the human genome. 41% of the total binding sites were identified on chromosomes 1, 19, 5, 2, 3, 14, 7 and X. Although the number of miRNA binding sites correlated with the size of each chromosome, some short chromosomes, such as 19 and X, had more miRNA binding sites when compared to other larger chromosomes (Table 4).
CHR= Chromosome.
14.89% (302) of binding sites grouped into the following 19 specific chromosomal locations: (1) 19q13.42 (51 sites/14 miRNAs), (2) 14q32.31 (34 sites/16 miRNAs), (3) 13q31.3 (16 sites/11 miRNAs), (4) 14q32.2 (16 sites/9 miRNAs), (5) 4q25 (16 sites/7 miRNAs), (6) 20q13.33 (15 sites/7 miRNAs), (7) 16p13.3 (15 sites/4 miRNAs), (8) Xq26.2 (14 sites/8 miRNAs), (9) 7q22.1 (14 sites/6 miRNAs) and (10) 1p31.3 (14 sites/6 miRNAs). The remaining 9 chromosomal locations contained between 10 and 13 binding sites (Supplementary File 2). 92% (1865/2028) of the binding sites were distributed into 250 groups along the human genome; the remaining 8% (163/2028) of binding sites for various miRNAs including miR-5095 were distributed along the human genome without being distributed into any groups.
Each group contains between 2 and 7 miRNA binding sites, although some groups contain between 8 and 16 (Figure 4). The majority of the groups are located on chromosomes 1, 2, 3, 5, 10 and 11. The biggest groups are located on chromosome 19, with 51 binding sites for 25 miRNAs involved in CC development.
58.8% of miRNA binding sites associated with CC (1194 binding sites) are located in intergenic regions, 39.65% (804 binding sites) in intronic regions, 1.28% (26 binding sites) in exonic regions and 0.19% (4 binding sites) between intronic and exonic regions (mixed miRNAs). Figure 5 shows the variation in the number of intergenic, exonic and intronic miRNAs associated with CC.
Thirty-eight integration sites were found for six types of oncogenic HPV (HPV-16, -18, -33, -45, -58 and -68) in miRNA binding sites and cell cycle regulatory genes associated with CC (Table 5). The largest number of HPV integration sites was found for miR-5095 (33 sites), followed by miR-548c-5p (11 sites) and miR-548d-5p (11 sites) (Table 5). In 14 integration sites, no miRNA binding sites were detected. The highest number of miRNA binding sites was found in chromosome regions 18q11.2 and 19p13.12 (Supplementary File 2).
| HPV TYPES | HPV INTEGRATION SITES | miRNAs PRESENT AT SITES OF INTEGRATION OF HPV1 | CELLULAR GENES2 | CL.3 |
|---|---|---|---|---|
| 18 | 1p22.2 | miR-548c-5p (-) | CDC7 (+) | -- |
| 18 | 1p31.2 | - | GADD45A (+) | ST |
| 16 | 1p34.1 | - | PLK3 (+) | -- |
| 16 | 1p34.3 | miR-5095 (3; -,-,+), -548b-5p (-), -548c-5p (2, -,-), -548d-5p (-) | CDCA8 (+) | OG |
| 16 | 1q25 | - | TPR (-) | -- |
| 16 | 1q36.32 | - | TP73 (+) | ST |
| 16,18 | 1q41 | miR-5095 (2,+,+), -194-5p (-), -215-3p (-), -215-5p (-), -548b-5p (-) | PROX1 (+) | ST |
| 18 | 2p15 | miR-5095 (-) | XPO1 (-) | ST |
| 16 | 2q33.1 | miR-152-5p(-), -548d-5p(-) | ORC2 (-) | -- |
| BZW1 (+) | -- | |||
| 16 | 2q33.3 | miR-5095 (+) | PARD3B (+) | ST |
| 16 | 2q34 | miR-5095 (-) | BARD1 (-) | ST |
| 16 | 3p21.31 | miR-5095 (3;-,+,+), -191-3p (-), -191-5p (-), -425-3p (-), -425-5p (-) | MAP4 (-) | -- |
| 16 | 3q26.33 | miR-5095 (2; -,+) | SOX2 (+) | OG |
| 16 | 3q28 | miR-5095 (-), -944 (+), -28-3p (+), -28-5p (+) | P3H2 (-) | ST |
| TP63 (+) | ST | |||
| 16, 45 | 4q13.3 | - | CXCL8 (+) | PO |
| 16 | 4q23 | - | EIF4E (-) | OG |
| 16 | 4q31.21 | miR-548c-5p (+) | FBXW7 (-) | ST |
| 16 | 5q11.2 | miR-5095 (3; -,-,+), -449a (-), -449b-3p (-), -449b-5p (-), -548c-3p (+), -548d-5p (+), -581 (-) | MAP3K1 (+) | ST |
| 16 | 5q31.1 | miR-5095 (-) | PPP2CA (-) | ST |
| 16 | 6p21.31 | miR-5095 (+) | BAK1 (-) | ST |
| 16 | 6p22.3 | miR-5095 (4; -,-,+,+), -548c-5p (+), -548d-5p (2; +,+) | ID4 (+) | ST |
| 16 | 6q22.32 | - | CENPW (+) | -- |
| 16 | 6q23.3 | miR-5095 (3; -,+,+) | CITED2 (-) | ST |
| 16 | 7p21.1 | - | AHR (+) | ST |
| 18 | 7q36.2 | miR-5095 (-) | RHEB (-) | PO |
| 18 | 8q21.2 | - | E2F5 (+) | -- |
| 16, 18 | 8q21.3 | - | NBN (-) | ST |
| 16, 18, 45 | 8q24.21 | miR-5095 (-), -548d-5p (-) | MYC (+) | PO |
| 16 | 8q24.21 | miR-5095 (-), -548d-5p (-) | PVT1 (+) | OG |
| 18 | 9p21.3 | miR-5095 (+), -31-3p (-), -31-5p (-), -491-3p (+), -491-5p (+) | CDKN2A (-) | ST |
| 16 | 9q22.2 | miR-5095 (+), -576-3p (2; +,+) | CKS2 (+) | OG |
| 16, 18 | 10q23.31 | miR-5095 (-), -107 (-), -103a-3p (-), -548b-5p (2; -,-), -548d-5p (2; -,-) | PTEN (+) | ST |
| 16 | 10q24.2 | miR-5095 (-), -1287-5p (-) | MARVELD1 (+) | ST |
| 16 | 12q14.3 | miR-574-5p (-) | CDK4 (-) | OG |
| MDM2 (+) | OG | |||
| 18 | 12q15 | - | HMGA2 (+) | PO |
| 58 | 12q24.33 | - | ZNF268 (+) | ST |
| 18 | 14q11.2 | miR-5095 (+), -548c-3p (+), -574-5p (+) | HAUS4 (-) | -- |
| 18, 45 | 14q24.1 | miR-5095 (2, -,+), -548c-5p (+) | RAD51B (+) | ST |
| 18 | 15q21.3 | miR-5095 (2; -,+), -574-5p (-) | CCNB2 (+) | PO |
| 16 | 16p13.3 | miR-5095 (12; (7 -, 5+,)), -548c-5p (+), -572 (-), -940 (+) | TSC2 (+) | ST |
| 16 | 17q21.31 | miR-5095 (3; -,+,+) | BRCA1 (-) | ST |
| 33 | 18q11.2 | miR-5095 (-), -1-3p (-), -133a-3p, -133a-5p (-), -133b, -378a-3p (+), -548b-5p (-), -548d-5p (-) | TTC39C (+) | -- |
| 68 | 18q21.1 | miR-5095 (3; -,+,+), -548c-5p (+), -548d-5p (+), -574-5p(+) | ZBTB7C (-) | ST |
| 18 | 18q21.33 | miR-5095 (-), -548b-5p (+), -548c-5p (-), -548d-5p (+) | BCL2 (-) | PO |
| 16 | 19p13.12 | miR-5095 (-), -23a-3p (-), -23a-5p (-), -27a-3p (-), -27a-5p (-), -181c-3p (+), -181c-5p (+), -584-5p (+) | NANOS3 (+) | -- |
| 16 | 20q11.21 | - | TPX2 (+) | ST |
| 16 | 20q13.2 | miR-5095 (-) | SRC (+) | PO |
| 16 | 21q22.13 | miR-5095(+), -548d-5p (-) | DYRK1A (+) | -- |
| 16 | 22q12.1 | miR-548c-5p (+) | CHEK2 (-) | ST |
| 16, 18, 45 | 22q13.1 | miR-5095 (2, -,-) | MCM5 (+) | PO |
| 16 | Xq25 | miR-5095 (-), -574-5p (-) | DCAF12L2 (-) | OG |
1The information in brackets shows the number of miRNA binding sites, and whether the miRNAs are located on the positive sense or negative sense DNA strand.
2The information in brackets shows and whether the cell cycle regulatory genes are located on the positive sense or negative sense DNA
3 Cl: Classification of cellular genes; ST: tumor suppressors; OG: Oncogenes; PO: Proto-oncogenes.
Ninety-six possible interactions were identified between 37 mature miRNAs associated with CC and 42 cell cycle regulatory genes located in proximity to the viral insertion sites. The network of interactions is presented in Figure 6. 35.42% of the interactions involved miR-5095, 12.5% involved miR-548c-5p and 12.5% miR-548d-5p.
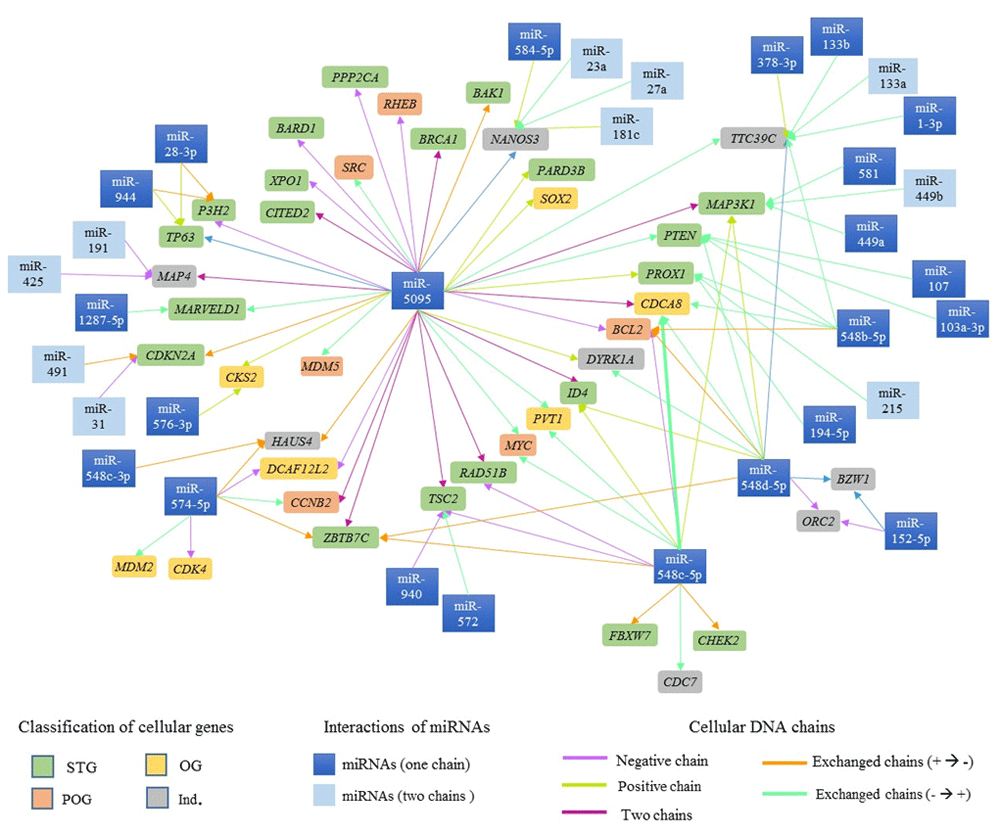
Rectangles of various colors represent the cell cycle regulatory genes, and color depend on their classification (ST -  , OG -
, OG -  , POG -
, POG -  e IND -
e IND -  ). The arrows represent the interactions between miRNAs and genes involved in cell cycle regulation; each arrow's color depends on the DNA chain where miRNAs and cell cycle regulatory genes are located.
). The arrows represent the interactions between miRNAs and genes involved in cell cycle regulation; each arrow's color depends on the DNA chain where miRNAs and cell cycle regulatory genes are located.
38.1% of genes identified in HPV integration sites have binding sites for a single miRNA, and 61.9% have binding sites for more than two miRNAs. Table 6 displays genes with more than five miRNA binding sites.
A gene may have binding sites for both regions of complementarity (3' and 5') of a miRNA38. In this study, we found that the TTC39C gene has binding sites for miR-133a-3p and miR-133a-5p and MAP3K1 has binding sites for miR-449b-3p and miR-449b-5p, though some mature sequences from one miRNA also showed binding sites to different genes (Figure 6). As an example, the miR-548c-3p mature chain has binding sites in the HAUS4 gene as well as in the MAP3K1, CDCA8, BCL2, ID4, cMYC, RAD51B, TSC2, ZBTB7C, FBXW7, CHEK2 and CDC7 genes (Figure 6).
26.31% (10/42) of the miRNAs analysed (miR-11-3p, miR-31-3p, miR-107, miR-133a-3p, miR-133a-5p, miR-133b, miR-215-5p, miR-491-3p, miR-548d-5p and miR-944) were identical across the Latin American human genome variants, and 73.69% showed a genetic mutation (substitution or deletion of nucleotides) (Figure 7, Panels A and B).
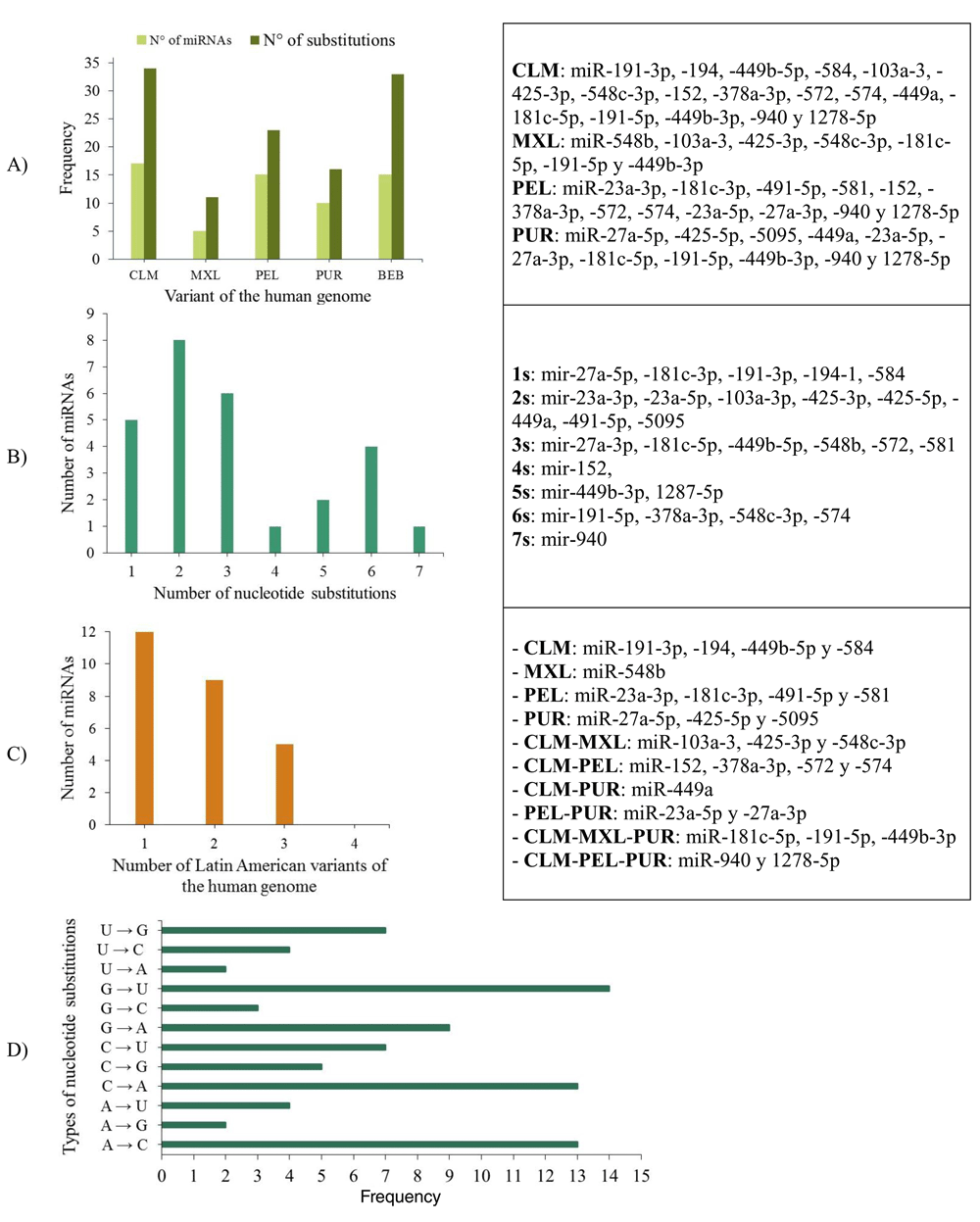
A) Number of miRNAs and nucleotide substitutions found in each human genomic variant; B) Number of miRNAs with between 1 and 7 nucleotide substitutions; C) Number of miRNAs with nucleotide substitutions in one, two or three genomic variants in the Latin American human genome, and D) Types of nucleotide substitutions in the miRNA sequences associated with CC in the selected human genome variants.
When mapping the sequences of these miRNAs to the selected Latin American human genome variants (Supplementary File 3), 88 miRSNPs related to miRNAs or miRNA binding sites were identified on the Latin American variants compared with 33 on the reference variant. Twenty-one miRSNPs were located in the miRNA seed sequences of Latin American variants compared with 3 located in the reference variant. The most representative mapping results are shown in Table 6.
Types of nucleotide substitutions in the miRNA sequences associated with CC in the selected human genome variants showed that there were more frequent transversions than transitions and that the most frequent nucleotide substitutions were G→U (16.9%), followed by A→C (15.7%), C→A (15.7%) and G→A (10.8%) (Figure 7).
Between one and 18 nucleotide deletions were detected in miR-27a-3p, miR-31-5p, miR-103a-3p, miR-191-3p, miR-215-3p and miR-574. The sequences of miR-28, miR-152, miR-548c-5p, miR-572 and miR-5095 only mapped to reference sequences (version GRCh38/hg38), but not to any of the Latin American human genomic variants. miR-152 did not map to the PUR variant (Table 6).
Table 7 displays the nucleotide variations from human genome variants obtained from Colombia, Mexico, Peru and Puerto Rico and Bangladesh, which was the control variant.
More data is available in Supplementary File 3.
| HG1 | miRNAs IDENTIFIED IN HPV INTEGRATION SITES (Cromosomal location (Chain))2 | |
|---|---|---|
| hsa-mir-1-3p (18q11.2 (-)) | hsa-mir-23a-3p (19p13.12 (-)) | |
|
CLM
MXL PEL PUR BEB |
UGGAAUGUAAAGAAGUAUGUAU UGGAAUGUAAAGAAGUAUGUAU UGGAAUGUAAAGAAGUAUGUAU UGGAAUGUAAAGAAGUAUGUAU UGGAAUGUAAAGAAGUAUGUAU | AUCACAUUGCCAGGGAUUUCC AUCACAUUGCCAGGGAUUUCC AUAACAUUGCAAGGGAUUUCC AUCACAUUGCCAGGGAUUUCC AUCACAUCGCCAGGGAUUUCC |

|

| |
| Conserved | Nucleotide substitution | |
| hsa-mir-31-5p (9p21.3 (-)) | hsa-mir-152 (17q21.32 (-)) | |
|
CLM
MXL PEL PUR BEB | AGGCAAGAUGCUGGCAUAGCU AGGCAAGAUGCUGGCAUAGCU AGGCAAGAUGCUGGCAU AGGCAAGAUGCUGGCAUAGCU AGGCAAGAUGCUGGCAUAGCU | CGGGUCUGUGCUACACUCCGACU CGACU AGGUUCUGUGAUACACUACGACU AGGUUCUGUUGUGCACUCUGACU |

|

| |
| Nucleotide deletion | Absence of the miRNA sequence | |
1HG: Human genome; CLM: variant of Medellin, Colombia; MXL: Los Angeles with Mexican ancestry; PEL: Lima, Peru; PUR: of Puerto Rico; BEB: Bengali, Bangladesh.
2The size of each letter indicates the enrichment of each nucleotide in Latin American variants of the human genome, displayed through WebLogo.
According to the literature, approximately 570 integration sites have been identified for eight oncogenic HPV types associated with CC (Figure 2). HPV integration into cellular DNA and consequent deregulation of genes is considered a crucial step in cancer progression. Genotype HPV-16 is the most studied for its relationship with CC, as it is responsible for 70% of cases worldwide39. This could be a consequence of the greater proportion of integration sites reported for this genotype. In contrast, low risk genotypes, such as HPV-45, -66 and -93 reported in Colombia, are frequent in CC40–44.
HPV integration into the host genome occurs in regions well-known as fragile sites, breakpoints or transcriptionally active regions45. This integration induces functional alterations of cellular genes in close proximity12,46–48. According to our results, the 8q24.21 chromosome region is the most affected by HPV integration. If we take into account that proto-oncogenes such as the MYC gene are located here49 (as displayed in Figure 3) and that MYC represents a family of genes overexpressed in several tumours including CC49–51, inhibition of MYC expression can induce cancer cell destruction50. In this context, the MYC gene could be both a tumour biomarker and potential treatment target for several tumours51 (Table 2).
Chromosomes 1, 14, 19 and X contain significantly more mature miRNAs than others, and chromosome 18 contains fewer miRNAs. The 19q13.4 chromosome region contains the largest group of human miRNAs (known as the group of miRNAs on chromosome 19 "C19 MC"), with alterations in several that have been previously reported in cancer52. Studies have reported associations between chromosome 1 and malignant transformation in cancers, including CC53.
The 578 integration sites identified in eight HPV types associated with CC were located in cell cycle regulatory genes, including the tumour suppressor genes TP73, P3H2, TP63, NBN, PTEN, BRCA1, and TPX2; the oncogenes EIF4E, CDCA8, MDM2, and PVT1; and the proto-oncogenes SRC, MYC, MCM5, CXCL8, and BCL2. Their deregulation could explain the progression of CC (Figure 3).
In 2011, Reshmi et al. used BLAT to determine the exact location of four miRNA binding sites associated with CC using bioinformatics programmes and computational tools54. To the best of our knowledge, this study is the first to use BLAT to identify miRNA binding sites in proximity to HPV integration sites involved in CC progression. In this study, 2028 binding sites from 272 CC-associated miRNAs were identified.
Identification of the target mRNAs of these miRNAs is considered a key step in their structural and functional analysis to establish possible interactions and consequently, cellular processes that may be altered in CC progression55–57. miRNAs located in the two strands of cellular DNA (5’ and 3’ strands) demonstrate their ability to interact in both orientations with the two strands of DNA and form triple helix structures to enhance RNA stability58,59.
Each CC-associated miRNA showed a different number of binding sites in the human genome (Table 3, Supplementary File 2), and in the human genomic variants17,21,60,61; miRNAs were distributed throughout the genomes in both intronic or exonic regions13. In this study, CC-associated miRNAs were distributed in the karyosome, with chromosomes 1, 19, 5, 2, 3, 14, 7 and X having the largest number of miRNA binding sites (Table 4). These results are consistent with those reported by Calin et al.12. Because some chromosomes have a greater number of miRNA binding sites, it provides evidence of a non-random distribution of miRNAs within the chromosomes.
Our results showed a low number of exonic miRNAs. These exonic miRNAs are considered rare miRNAs62, which are important candidates for gaining a better comprehension of interaction networks between miRNAs and their CC-associated targets.
The miRNA binding sites are within a short distance of each other in the chromosome, indicating that they tend to cluster63–66. Altuvia et al. reported miRNAs in groups of two or three64. This coincides with our results on CC-associated miRNA binding sites, as we found that miRNAs are capable of forming groups of more than 6 miRNAs on both strands of human DNA (Figure 4). We identified an important group of 16 miRNAs that can form these clusters and are located on chromosome 14 region 14q32.31. They include hsa-miR-134, miR-299, miR-323a, miR-329, miR-376a, miR-376c, miR-379, miR-411, miR-485, miR-487a, miR-487b, miR-494, miR-495, miR-539, miR-654 and miR-5095 (Supplementary File 2). Understanding their individual and collective roles is important when studying the development of this neoplasia.
miR-5095 had the highest number of binding sites distributed throughout the human genome (Table 3), which is in accordance with previously reported data66–68 where approximately 900 binding sites were identified; they are probably related to the expression of many target mRNAs and biological processes. Based on its extensive genomic distribution and low specificity in CC, miR-5095 is a good candidate to be used as an indicator of genetic variability within the human population.
To identify the role of miRNAs, HPV integration sites located in cell cycle-controlling genes were analysed. Thirty-seven miRNAs were identified in HPV integration sites close to cell cycle-controlling genes (Table 5). Nambaru et al. and Schmitz et al. identified numerous miRNAs in the proximity of HPV integration sites and reported that approximately 65% of these were involved in cervical carcinogenesis8,9. Inactivation of tumour suppressor genes by viral integration increases genomic instability and leads to cervical malignant neoplasm progression69.
The multiple miRNA binding sites on a target may decrease the levels of mRNA translation and improve the specificity of gene regulation. For example, one miRNA can have multiple target genes and each individual mRNA can be regulated by numerous miRNAs13,70,71. Ninety-seven interactions were identified between miRNAs and cell cycle regulatory genes (Table 4–Table 5, Figure 4–Figure 6); miR-5095, -548c-5p and -548d-5p showed the highest number of interactions with these kinds of genes.
Ivashchenko et al. identified miR-5095 binding sites in the BRCA1 gene67. In this study, miR-5095 was also found to have binding sites in the BAK1, BARD1, CITED2, MDM5, SRC, PARD3B, PPP2CA, RHEB, SOX2 and XPO1 genes (Table 5 and Figure 6). Our findings provide a basis for searching for other interactions, gene targets, and CC-associated miRNAs.
During miRNA biogenesis, each pre-miRNA produces two mature miRNAs, such as miRNA-5p and miRNA-3p72. Mature miRNA deregulation can have an important role in tumour development, suggesting the need to analyse each mature sequence (miRNA-5p and -3p). In this study, binding sites were analysed for both mature miRNA sequences (-5p and -3p) in several interactions (Figure 6). A mature miRNA sequence, such as miR-548c, demonstrated binding sites in different cellular genes. Thus, this miRNA could serve as candidate biomarker for CC prognosis and diagnosis.
Han et al. characterized the two mature chains of miR-21 and their oncogenic roles in cervical cancer73. The regulation of the mature 5p and 3p chains from several miRNAs has been investigated in other cancers, including colorectal, gastric, breast, lung, kidney, and bladder36,72,74–77, suggesting the need to focus further studies on the two mature chains from the 272 miRNAs reported in this study.
Figure 6 shows the complexity of the interactions between miRNAs and tumour suppressor genes, proto-oncogenes and oncogenes. The study of interaction networks between cell cycle genes and miRNAs involved in cancer is one of the most recent challenges in systems biology and is important for elucidating the control mechanisms for cancer biological process78–81.
The differences in miRNA expression profiles between normal and cancerous tissues have led to the identification of clinical biomarkers for the early detection of many diseases, including various cancers and their precursor stages79,82,83. Research on miRNAs associated with cancer has not taken into account the genetic variability in human populations, which influences the structure, expression and function of miRNAs in populations from different ethnic backgrounds. Studies on genetic variability are relevant to designing strategies for the diagnosis and prognosis of various diseases.
miR-11-3p, miR-31-3p, miR-107, miR-133a-3p, miR-133a-5p, miR-133b, miR-215-5p, miR-491-3p, miR-548d-5p and miR-944 were conserved in the four human genome variants. In the remaining 27 miRNAs, substitutions, deletions or insertions were observed in the nucleotide sequences, indicating that this variability can be decisive when determining susceptibility to the development of CC (Table 7 and Supplementary File 3).
There are numerous studies that analyse miRSNPs in different malignancies84–86, but there is no available data on the correlation of SNPs in CC-associated miRNAs located in HPV integration sites in Latin American human genomic variants.
According to our results, the genomes from Latin America showed a lower miRSNP frequency compared to the control genome (BEB), although the Colombian (CLM) genome frequency was more similar to the BEB genome. Latin American populations have experienced migrations from European, Asian and African individuals87. Thus, our results could be a result of the specific interracial mixing of Colombian populations but also due to migration patterns during human settlement in Latin America.
miRSNPs can affect the structure and function of miRNAs by impacting interactions between miRNAs and their mRNA targets or interfering with the expression levels of individual miRNAs20–22,88,89. miRSNPs could cause the loss or gain of binding sites for the co-evolution of miRNAs and their target mRNA and even influence cell processes related to tumour progression, disease phenotypes or susceptibility to developing a specific disease.
More studies are needed to clarify the role, targets and transcriptional regulatory mechanisms of cellular events in which miRNA are involved, including differentiation, apoptosis, metabolism and carcinogenesis. The expression and deregulation of miRNAs in cancer as well as their role as biological markers in diagnosis and treatment of CC should be explored. Further identification of cellular genes and signalling pathways involved in CC progression could lead to the development of new therapeutic strategies based on miRNAs90,91. Additional biomarkers associated with apoptosis, necrosis and possible interactions with CRISPR complex sequences can be explored in order to develop therapeutic strategies in the future.
Dataset 1. The mature miRNA reference sequences were obtained in FASTA format from the miRBase database. DOI, 10.5256/f1000research.10138.d16473228
Dataset 2. Matrix of data containing all the necessary components for the validation of data on CC-associated miRNAs in HPV integration sites in Latin American human genomic variants. DOI, 10.5256/f1000research.10138.d16473636
MGF directed all the research and bioinformatics analysis, wrote the article and made the final edits. OAGG developed the methodology and bioinformatics analysis and edited the article. JMH co-advised the research, wrote the article and made the final edits, and MCYC wrote the article.
The authors thank the recommendations and suggestions of cPhD. Guillermo Torres from Kiel University (Germany) to improve the bioinformatics approach in this research.
Supplementary File 1. Articles that mention HPV integration sites, detailing the most frequent types of HPV associated with CC.
Click here to access the data.
Supplementary File 2. Diagram indicating the regions on all chromosomes with miRNA binding sites that are associated with cervical cancer.
Click here to access the data.
Supplementary File 3. miRNAs identified in HPV integration sites, displaying the nucleotide variations in the selected Latin American human genome variants and in the control variant.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Mullany LE, Herrick JS, Wolff RK, Slattery ML: MicroRNA Seed Region Length Impact on Target Messenger RNA Expression and Survival in Colorectal Cancer.PLoS One. 2016; 11 (4): e0154177 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 05 Dec 18 |
read | read |
|
Version 1 20 Jun 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)