Keywords
Granular cell tumor, skin, atypical type
Granular cell tumor, skin, atypical type
Granular cell tumor (GrCT) is a benign tumor of the nerve sheath1, which more commonly occurs in the tongue, breast, skin and subcutis. It can affect the dermis, subcutis or submucosa. Granular cell tumors are uncommon tumors and most of them have a good prognosis after surgical resection, however, around 0.5–2% of these tumors may be malignant, which have a poor prognosis due to local recurrence and distant metastasis1–3.
Granular cell tumors are rare in the deep soft tissues of the extremities, especially those of intramuscular origin2–5. Although this type of tumor is more common in the 4th to 6th decades of life, one study found that GrCT occurs more commonly between 30–40 years of age1. In this case report, we present a 30 year old man with a skin lesion, which was diagnosed as a granular cell tumor.
A 30 year old man was referred to the pathology department of the Imam Hospital, Sari, Iran in August 2017, and presented with a skin lesion on his right shoulder (Figure 1). He had no pain or trauma, and no significant past medical history. The patient had the skin lesion for 6 months previous to presentation, which had grown slowly in size over the course of previous 2 months. The lesion size was approximately 2 cm in diameter with a verrucous appearance. On physical examination, a hard, fixed and non-tender skin mass was palpable on his right shoulder.
Complete tumor excision was performed and histopathological findings revealed a marked hyperplasia epidermis with pseudoepitheliomatous pattern (Figure 2A). The dermis showed ill-defined and diffuse proliferation of large round to oval cells, with brightly eosinophilic granular cytoplasm (Figure 2B). Mitotic activity was rare. Atypia and necrosis was not seen. The pathologic report was compatible with a granular cell tumor.
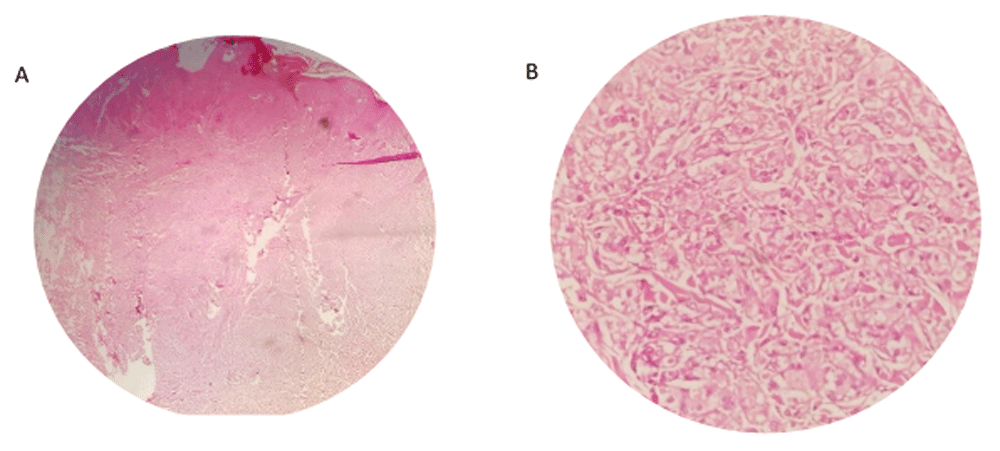
A - Hematoxylin and eosin (H & E) staining of tissue sample under ×40 magnification showing pseudoepitheliomatous hyperplasia of epidermis. B - H & E staining of tissue sample under ×400 magnification showing large granular cell.
Immunohistochemical (IHC) staining was carried out for this patient in the pathology department of Imam Khomeini hospital. All samples were fixed in 10% buffered formalin and embedded in paraffin. Sections were cut 4 μM thick from wax blocks, mounted on to 3-Aminopropyltriethoxysilane (APES)-coated glass slides. Slides were deparaffinized in xylene twice for 10 minutes, rehydrated through graded ethanol to distilled water before incubation for 15 minutes with 3% hydrogen peroxidase-methanol to inhibit endogenous peroxidase activity, and heated in 0.01 M citrate buffer (pH 6.0) in a microwave oven for 5 minutes at 100°C; after boiling for antigen retrieval. Then the slides were taken out of microwave oven and cooled to room temperature for 30 minutes. After incubating for 15 minutes in a blocking solution containing 10% normal goat serum in PBS, sections were incubated at 4°C overnight in a humidified chamber with CD68, S100, neuron specific enolase (NSE), vimentin, Ki67, desmin and SMA antibody6. The prepared stained slides were read using Olympus CX31 microscope.
Periodic acid–Schiff stain (PAS) was positive in the suspected tumor cells, and IHC results showed, CD68 (Manufacturer No. Mob167, species: mouse, clone ID No: kp1, concentration: 1:100; CellPath Ltd, UK), S100 (Manufacturer no. Z 0311, species: rabbit, clone ID No: polyclonal, concentration: 1:500; Agilent, Santa Clara, CA, USA), NSE (Manufacturer no. RP 054, species: rabbit, concentration: 1:50; CellPath, UK), and vimentin (Manufacturer no. Mob 090, species: mouse, clone ID No: v9, concentration: 1:50; CellPath, UK), were strongly positive (dark brown staining +3) in suspected cells (Figure 3-A, B, C) and Ki67 (Manufacturer No. DB D-125, clone: C16-I, species: rabbit, concentration: 1:200; DB Biotech, Kosice, Slovakia) was positive in 3% of tumor cells. Desmin and SMA were negative (Figure 4- A, B). Finally, the granular cell tumor was confirmed and the patient has been followed for 1 year after the surgery and no recurrence has been reported.
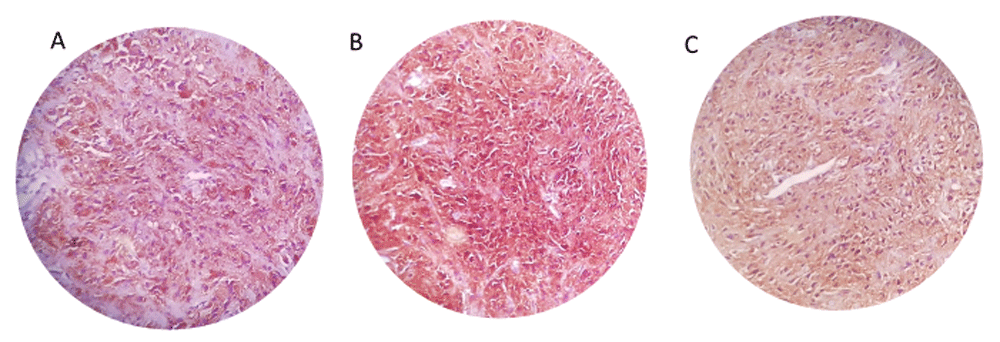
Immunohistochemistry study positive staining of tissue samples for NSE (A), CD68 (B), S100 (C).
GrCT was first described in 1926 as a myoblastoma which arises from the muscle in the tongue. Apart from the tongue, the skin and soft tissues are other common locations for GrCTs1. In 1935, Feyrter described the tumor as a granular cell neuroma because he hypothesized that the tumors were neural in origin. Fust and Custer named the tumor as granular cell neurofibroma in 1948. Finally in 1962, Fisher and Wechsler named the tumors as granular cell schwannomas, because Schwann cells was their most probable origin. Nowadays the name adopted by WHO is granular cell tumor7. GrCT usually presents as a solitary and small nodule, as a painless mass. It is most common in women aged 30–60 years old1,8. The presented case had a painless mass and as a 30 year old male, he did not conform to epidemiological evidence on the most common sex9. Furthermore, based on the clinical findings, the dermatologist diagnosed this lesion as dermatofibroma and keratoacanthoma and no differential diagnosis had been reported. However, the pathology report showed different results and identified it as granular cell tumor.
To our knowledge, three similar cases of GrCT with distinguished dermatofibroma-like morphology have been described in the literature. However, all these cases were presented as atypical GrCT. One was a 60 year old woman with a nodule on the back10, the second was a 48 year old man with a lesion in the pubic area11 and the third case was a 62 year old woman with a tumor on her back under the right scapula12.
According to 6 histological criteria, GrCT can be classified as benign, atypical or malignant. The criteria are necrosis, spindling, vesicular nuclei with large nucleoli, increased mitotic activity (>2 mitoses/10 high-power fields), high nuclear to cytoplasmic ratio, and pleomorphism1. Tumors with 1 or 2 of these criteria can be classified as atypical. Another classification system states the only difference between benign GrCT and GrCT-uncertain malignant potential is the presence of necrosis and/or mitoses13. As to the former classification system, there are some cases of histological mild atypic GrCTs, which presented a malignant clinical course such as local recurrence, rapid recent growth, and large tumor diameter14.
GrCTs are relatively uncommon and benign in most of the cases1,15. Malignant and atypical GrCTs account for only a small percentage of cases1. The most common immunological marker presented by GrCT is S100 protein. In our case, we excluded possible diagnosis of granular cell dermatofibroma (S100-protein negative) and malignant peripheral neural sheath tumor (weak S-100 expression). CD68, CD57, and NSE may be positive in GrCT cases1.We can evaluate malignant potential in GrCTs by the means of Ki-67 proliferation index. If the index is greater than 10% in a specific case, the malignant potential is higher in that case, although not all malignant GrCTs have a high Ki-67 index. In addition to immunological markers mentioned above, it is shown that 68% of GrCTs express p53 in over 50% of tumor cell nuclei1.
In conclusion, we presented a case of GrCT with the dermatofibroma-like morphology fulfilling criteria of benign GrCT and immunhistochemical positivity of S100, CD68, and NSE. The necessity for S-100 staining to differentiate granular cell tumor with dermatofibroma from dermatofibroma-like GrCT is highly recommended.
Written informed consent for the publication of the patient’s clinical details and images was obtained from the patient.
Dataset 1: Raw microscope images 10.5256/f1000research.13015.d20701016
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical oncology, DFSP, granular cell tumors, melanoma, sarcoma, squamous cell carcinoma, merkel cell carcinoma, colon cancer, rectal cancer, gastric cancer, neuroendocrine tumors.
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Jul 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)