Keywords
Innate Immune Receptor NLRX1, Lower and Upper UTI, Pyelonephritis, Animal model, Escherichia coli
This article is included in the Pathogens gateway.
Innate Immune Receptor NLRX1, Lower and Upper UTI, Pyelonephritis, Animal model, Escherichia coli
We updated paper to address the important points raised by the reviewers. We furthermore added Supplementary Figure 3 and underlying data with the kinetics of the urophatogenic E. coli strain outgrowth in WT mice at 4,8,24 and 48h in the bladder as requested by the reviewer.
See the authors' detailed response to the review by Sheryl Justice
See the authors' detailed response to the review by Sylvia Knapp
Toll like receptors (TLRs) and NOD-like receptors (NLRs) are members of a large family of extracellular and intracellular pattern recognition receptors (PRRs) that trigger immune responses to prevent pathogen invasion and growth1,2. Urinary tract infections (UTIs) are common bacterial infections in humans, that occur most commonly in women and children3. UTIs are caused by the presence of uropathogenic bacteria, usually Escherichia coli (E. coli), in the lower urinary tract (bladder) that overcome the host innate immune defense. When the infection ascends from the bladder via the ureters to the upper renal pyelum, lower UTI can lead to acute pyelonephritis. If untreated pyelonephritis can have serious implications for renal functioning and the development of damage and scarring4,5. Antimicrobial resistance among UTIs are increasing6,7, therefore new insights in host defense mechanisms are required to obtain new targets for therapy.
TLRs are known to play an important role in the host response to UTIs8, whereas the role of NLRs herein is unclear. NOD-like receptor X1 (NLRX1) is an ubiquitously expressed PRR in mitochondria that controls mitochondrial activity in tubular epithelial cells and hepatocytes, and in this way effects respectively ischemic acute kidney disease and liver steatosis9,10. Other functions for NLRX1 include negative regulation of antiviral immunity11, and inhibition of NF-κB signaling by disrupting interaction of TRAF6 and IKK12,13. Given these studies, NLRX1 could play a potential role during the pathophysiology of acute bacterial infections such as pyelonephritis.
To get more insight in NLRX1 functioning during bacterial infection we investigated in the present study the role of NLRX1 during uropathogenic E. coli-induced lower and upper UTI in mice. We found that although NLRX1 absence enhances bacterial burden in the bladder during the early phase of infection, NLRX1 is not involved in the host defense against pyelonephritis.
NLRX1 KO mice with a C57BL6/J background were generated as described previously14 and bred at the animal facility of the Academic Medical Center (AMC) in Amsterdam, The Netherlands. Age- and gender-matched C57BL6/J WT mice were obtained from Charles River (Maastricht, The Netherlands). The mice were allowed to acclimatize for a week in the same room and conditions as the transgenic animals before starting the experimental procedures. Animals were housed in individual ventilated cages (IVCs) with bedding and cage enrichment that were kept under standard environmental conditions (temperature, humidity, ventilation, light/dark cycle) and under specific pathogen-free conditions (SPF) with ad libitum access to water and food.
The in vivo and ex vivo experiments were carried out once and the data showed in the article is based on biological replicates. The in vivo study was performed with 2 experimental groups: 1) WT (n=8) and 2) NLRX1-KO (n=8) and 2 sham/control groups: 3) WT (n=4) 4) NLRX1-KO (n=4). Each experimental group was subjected for two time points (24h and 48h) to UTI as described previously15 and briefly explained later. The total number of mice per in vivo experimental group was 16 and the total number per sham/control group was 4. For the data obtained in Suplementary Figure 3 only WT mice (n=6-8 per time point) were subjected to UTI according the protocol described below, mice were sacrificed 4h, 8h, 24h and 48h post inoculation. To be able to reach a statistical significant effect of NLRX1 deficiency the number of 8 mice per experimental group was assessed with an unpaired t-test based on a variation coefficient of 15%, a minimal relative effect of 30%, a P value of 5% and a power of 80%, that were based on previous studies done in our group15–17. For both experimental and sham/control groups 11–12 week old female mice (median weights: WT; 19,6 and NLRX1-KO; 21,3 grams) were used. Each experimental group was divided in 2 cages of 3 and 5 animals and in the sham/control group 4 animals per cage were kept. For the experimental groups uropathogenic E. coli 1677, isolated from an uroseptic patient, was cultured in the laboratory in sterile Tryptic Soy Broth (TSB) overnight at 37°C, 5% CO2. The next day, in the morning this suspension was diluted 1:100 in fresh TSB and in 2–3h cultured to optical density OD620nm = 1 was reached (measured with a spectrophotometer (DU640, Beckman, USA)). Subsequently bacteria were spun down for 14 min at 4000 rpm at 4°C, washed three times and resuspended in 10 mL sterile PBS. The same day, in the animal facility, under general anaesthesia (10 µl/1 g body weight of FFM mixture, containing 1.25 mg/ml midazolam (Dormicum®, Roche, Woerden, The Netherlands), 0.08 mg/ml fentanyl citrate/2.5 mg/ml fluanisone (Hypnorm, Veta Pharma Ltd., Leeds, UK)) that was given intraperitoneal, mice were via the urethra intravesically inoculated with 8*108 CFU in a 100µl volume. Mice in the sham/control group underwent the same procedure with administration of 100µl sterile PBS. CFU concentrations in the inoculum were determined by plating 10-fold serial dilutions on blood-agar plates at 37°C, 5% CO2 overnight. Mice were sacrificed 24 and 48 hour post-inoculation by cardiac puncture under 4% isoflurane/O2 followed by cervical dislocation. Blood was collected in lithium-heparin containing tubes and kidneys and bladders were collected for further analysis. In the WT 24h group one animal reached, because of signs of severe sepsis, the humane end point and was excluded from further data analysis. The animals used to study leukocyte composition and ex vivo granulocyte and monocyte functioning contained 2 experimental groups: 1) WT (n=6) and 2) NLRX1-KO (n=6) and were sacrificed as described earlier for the in vivo experiments. The total number of mice per ex vivo experimental group. To be able to reach a statistical significant effect of NLRX1 deficiency the number of 6 mice per experimental group was assessed with an unpaired t-test based on a variation coefficient of 10%, a minimal relative effect of 30%, a significance of 5% and a power of 80%. Mice used for the ex vivo experimental groups were 13–14 week old female mice (median weights: WT;26.5 and NLRX1-KO;26.6 grams). In the WT group one sample was excluded from further data analysis due to low blood gain.
All animal procedures were ethically approved under DPA 25 AB-1 by the Animal Care and Use Committee of the Academic Medical Center Amsterdam and were conducted in compliance with the ARRIVE guidelines (NC3Rs).
Bladder 20% (w/v) and left kidney 10% (w/v) tissues were homogenized in sterile PBS with a tissue homogenizer (Polytron PT1300D homogenizer, Kinematica AG). To determine bacterial loads 10-fold serial dilutions of bladder- and kidney-homogenates were plated onto blood agar plates. Colonies were counted 16h after incubation at 37°C.
Absolute leukocyte number in blood was measured with a Coulter Counter (Beckmann Coulter Inc., Fullerton, CA). To assess relative leukocyte composition, 100 µl whole blood erythrocytes were lysed by adding 2 ml lysis buffer (8.3g/L NH4Cl, 1.0g/L KHCO3, 0.1 mM EDTA, pH 7.4) for 15 min at RT. The remaining cells, leukocytes, were washed once, centrifuged and resuspended in FACS buffer (0.5% BSA, 0.01% NaN3, 0.35mM EDTA in PBS) and were measured by flow cytometry on a FACS Canto (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo version 10 software. We applied a forward versus side scatter (FSC vs SSC) gating strategy on living cells as displayed in Supplemental Figure 2 to discriminate between lymphocytes, granulocytes and monocytes, although adding specific markers would have given a better discrimination of the different populations.
Whole blood of WT and NLRX1 KO mice was incubated at 37°C, 5% CO2 in 10ng/mL LPS (cat no. l4391, Sigma-Aldrich, Zwijndrecht, The Netherlands) conditioned RPMI medium (Thermo fisher Scientific, Waltham, MA, USA) containing 10% FCS (Invitrogen, Carlsbad, CA, USA) with 2 mM L-glutamine and 100 U/mL penicillin/streptomycin (all from Thermo fisher Scientific, Waltham, MA, USA). After 14h, cells were spun down (5 min, 4000 rpm) and supernatants were collected and stored at -20°C prior to use for cytokine measurements.
Phagocytosis by granulocytes and monocytes was measured with the PHAGOTEST (cat no. 10–0100, Glycotope Biotechnology, Heidelberg, Germany) according to the manufacturer’s instructions. Briefly, 100 µl of heparinized whole blood was incubated with opsonized FITC- labeled E. coli for 10 minutes in a 37°C water bath, whereas the negative controls remained on ice. After phagocytosis was stopped the surface signal was quenched. After lysing the erythrocytes and fixation of the leukocytes, phagocytic capacity was measured by flow cytometry on a FACS Canto (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo version 10 software according the gating strategy displayed in Supplementary Figure 218
Bladder 5% (w/v) and kidney 10% (w/v) tissues from 24h and 48h UTI subjected WT and NLRX1 KO mice were homogenized with a tissue homogenizer (Polytron PT1300D homogenizer, Kinematica AG) in Greenberger lysis buffer (GLB) (300mM NaCl, 30mM Tris, 2mM MgCl2, 2mM CaCl2, 1% (v/v) Triton X-100, pH set at 7.4, supplemented with Protease Inhibitor Cocktail II (cat no. p8340, Sigma-Aldrich, Sigma-Aldrich, Zwijndrecht, The Netherlands). Levels of keratinocyte-derived chemokine (KC), macrophage inflammatory protein-2 (MIP-2), monocyte chemoattractant protein 1 (MCP-1), interleukin 1β (IL1β), interleukin 6 (IL6), tumor necrosis factor alpha (TNFα) and mouse myeloid peroxidase (MPO) were determined in bladder and kidney homogenates and whole blood plasma (KC, TNFα and IL6 only) by duo set ELISAs (cat no. MKC00B, MM200, MJE00, MLB00C, M6000B, MTA00B, DY3667, R&D Systems, Abingdon, UK), performed according to the supplied protocols. ELISA data measured in bladder and kidney homogenates was adjusted for total protein concentration as determined by BCA protein assay (cat no. B9643, Sigma-Aldrich, Sigma-Aldrich, Zwijndrecht, The Netherlands) developed with 4% CuSO4.
From hepanarized blood, plasma was obtained from the upper phase after spinning the tube for 10 minutes at 2000 rpm. Urea and creatinine levels in plasma were determined at room temperature by colorimetric enzyme reactions involving creatinase and urease and analyzed on the Cobas c702 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN, USA) according to standard diagnostic procedures performed by the department of Clinical Chemistry of the Academic Medical Center Amsterdam.
Total RNA was extracted from snap-frozen -80°C stored bladder and kidney using TriReagent (cat no. T9424, Sigma-Aldrich, Zwijndrecht, The Netherlands) followed by chloroform phase separation to obtain the aqueous RNA containing upper phase and isopropanol precipitation according to the manufacturer protocol procedure description and converted to cDNA. cDNA was synthesized using M-MLV reverse transcriptase according the procedure described in the manufacturer protocol (cat no. 28025, Thermo Scientific). Transcription was analyzed by RT-qPCR on a Roche LightCycler 480 using 2.5 µl sensiFAST SYBR master mix (cat no. bio-98020, Bioline reagents, London, UK), 0.20 µl forward primer, 0.20 µl reverse primer (Table 1), 2.10 µl distilled H2O and 1µl cDNA per reaction. qPCR primers were synthesized by Eurogentec (Maastricht, The Netherlands) and described in the list below. qPCR data was analyzed based on linear regression using the LinRegPCR program, that is freely available19,20. Briefly, the LinRegPCR program imports non-baseline corrected qPCR data, performs a baseline correction on each sample separately, determines a window-of-linearity and then uses linear regression analysis to determine the PCR efficiency per sample from the slope of the regression line. The mean PCR efficiency per amplicon and the Cq value per sample are used to calculate a starting concentration per sample, expressed in arbitrary fluorescence units (au)20.
Data are expressed as mean ± standard error of the mean (SEM), bacterial outgrowth data are expressed on a logarithmic scale as median scatterplot. The non-parametric Mann Whitney U test was performed for two group comparison. For all analyses, values of P ≤ 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
To determine whether NLRX1 expression is modulated in the murine bladder and kidney during urinary tract infection (UTI), wild-type (WT) mice were intravesically inoculated with uropathogenic E. coli and sacrificed at 24h and 48h after infection. Non-infected sham mice were used as controls. Real-time quantitative PCR revealed that Nlrx1 transcript levels were constitutively present in the bladder and kidney (Figure 1A and B). Nlrx1 transcript levels show a non-significant trend towards increased levels in the bladder after 24h, while levels are returned towards baseline sham levels at 48h (Figure 1A). In the kidney Nlrx1 transcript levels remained at baseline level 24h after infection while after 48h levels were significantly increased (Figure 1B). Together, these data show that in response to UTI, local Nlrx1 expression is increased upon E. coli infection.
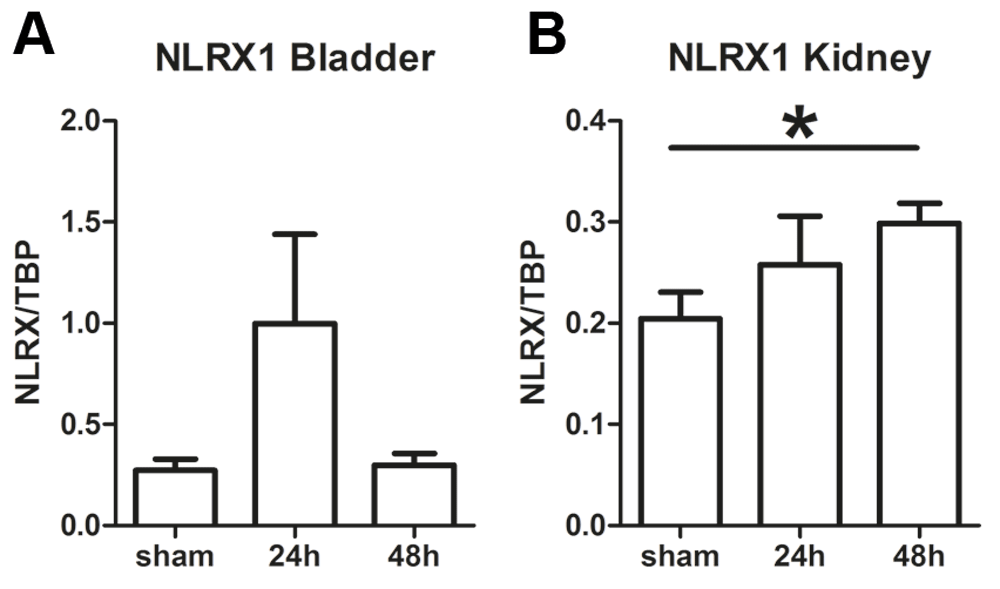
Nlrx1 mRNA transcript levels in wild-type (WT) (A) bladder and (B) kidney from sham, and uropathogenic E. coli-inoculated mice. All data are expressed as mean ± SEM, n=4 (sham) and n=7-8 (24h and 48h) animals per group. Statistical significance was determined by non-parametric Mann Whitney U test, *P<0.05.
To investigate whether NLRX1 plays a role in bladder and kidney during lower and upper UTI, we examined bacterial loads in these organs from WT and NLRX1 knock-out (KO) mice 24h and 48 h after inoculation with uropathogenic E. coli. This revealed that the bacterial outgrowth, as measured by colony forming units (CFU), in bladder tissue from NLRX1 KO mice was significantly higher at 24h after infection compared to WT while at 48h no differences in bacterial burden were found (Figure 2A). NLRX1 deficient mice had more improved bacterial clearance from the bladder at 48h as compared to 24h (Figure 2A). No differences in the amount of CFU were found between kidneys from WT and NLRX1 KO mice at both time points (Figure 2B). To monitor the local inflammatory response during infection, we next measured the levels of KC, MIP-2, MCP-1, IL-1β, IL-6 and TNFα in kidney and bladder homogenates (Figure 2C and D). The production of MIP-2 in the bladder was in NLRX1 KO mice reduced at both 24h and 48h after infection compared to WT animals (Figure 2C). In addition, NLRX1 KO bladders show reduced levels of MCP-1 and TNFα compared to WT at 48h, while no differences were found in KC, IL-1β and IL6 levels (Figure 2C). We identified an increase in renal KC levels at 24h in NLRX1 KO mice compared WT mice, whereas at 48h KC levels were similar between both groups (Figure 2D). At both 24h and 48h no differences were found in renal MIP-2, MCP-1, IL-1β, IL-6 and TNFα levels between WT and NLRX1 KO mice (Figure 2D). In addition, upper UTI and NLRX1 have no significant influence on renal function as reflected by similar plasma levels of urea and creatinine between all mice (Supplementary Figure 1A and B21). Accordingly, this indicates that in the bladder the lack of NLRX1 together with an impaired pro-inflammatory cytokine response is associated with an early impaired ability to clear uropathogenic E. coli. No differences were found in bacterial burden and cytokine response in the kidney when mice were deficient for NLRX1.

Outgrowth of uropathogenic E. coli expressed in colony forming units (CFU) in (A) bladder and (B) kidney homogenates from wild-type (WT) and NLRX1 knock-out (KO) mice 24h and 48h after inoculation. Detection limit: 10 CFU/ml. Levels of KC, MIP-2, MCP-1, IL-1β, IL6 and TNFα in (C) bladder and (D) kidney homogenates from the E. coli inoculated WT (white bars) and NLRX1 KO (black bars) mice. Data at A and B are expressed on a logarithmic scale as median scatterplot. Data at C and D are expressed as mean ± SEM. For all data n=7-8 animals per group and statistical significance between WT and NLRX1 KO was determined by non-parametric Mann Whitney U test, *P<0.05 and **P<0.01.
By analyzing inflammatory cells in the circulation we observed that the numbers of granulocytes and monocytes were equal between uninfected WT and NLRX1 KO mice (Figure 3A). A non-significant trend towards an increased presence of lymphocytes in NLRX1 KO compared to WT is shown (Figure 3A). We observed that WT and NLRX1 KO mice 24h and 48h after inoculation, have similar numbers of circulating leukocytes (Figure 3B). Recruitment of neutrophils in the kidney and bladder are essential for the host defense against uropathogenic E. coli22. Therefore, we determined active neutrophil presence by measuring kidney and bladder myeloperoxidase (MPO) concentrations. No differences in MPO levels were found in bladder and kidney between WT and NLRX1 KO mice at 24h and 48h (Figure 3C and D), indicating a similar number of activated neutrophils.
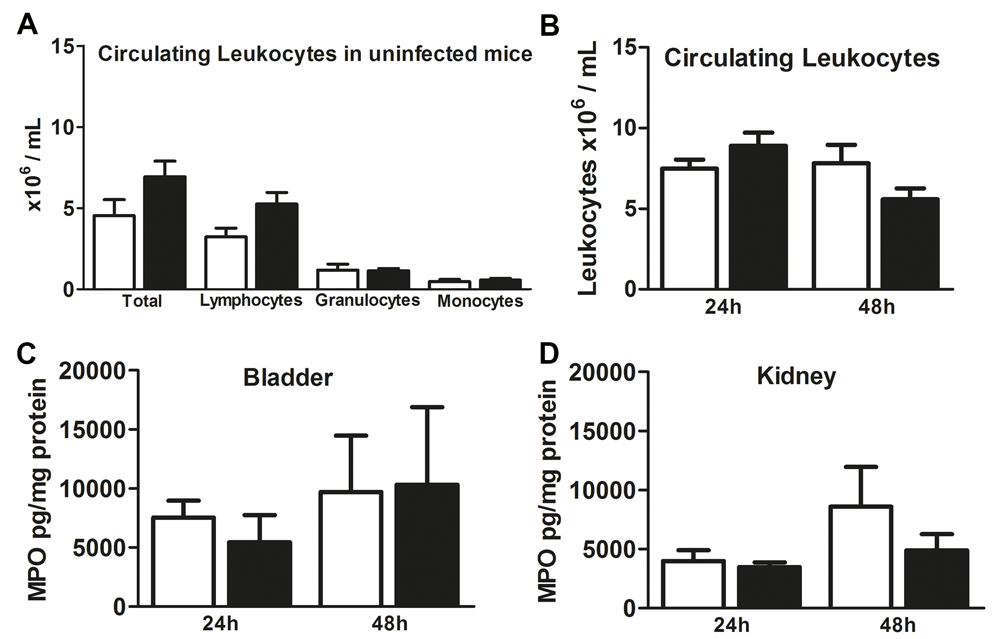
(A) Total circulating leukocyte, lymphocyte, granulocyte and monocyte cell counts in blood from uninfected wild-type (WT) (white bars) and NLRX1 knock-out (KO) (black bars) mice n=5–6 animals per group. (B) Circulating leukocytes in blood from WT (white bars) and NLRX1 KO (black bars) mice 24h and 48h after uropathogenic E. coli inoculation. MPO levels in (C) bladder and (D) kidney homogenates from 24h and 48h inoculated WT (white bars) and NLRX1 KO mice (black bars). All data are expressed as mean ± SEM, n=7–8 (B–D) animals per group. Statistical significance between WT and NLRX1 KO was determined by non-parametric Mann Whitney U test.
Since we observed differences in bacterial outgrowth in the bladder while the number of local neutrophils after infection is equal between WT and NLRX1 KO, we investigated if NLRX1 absence causes functional changes to granulocytes and monocytes. This revealed that NLRX1 is not critical for the secretion of the pro-inflammatory cytokines KC, TNFα and IL6 after ex vivo whole blood LPS stimulation (Figure 4A, B and C). To investigate if NLRX1 is important for the phagocytic activity of granulocytes and monocytes, leukocytes from WT and NLRX1 KO mice were ex vivo incubated with fluorescein labelled opsonized E. coli, and phagocytosis was analyzed using flow cytometry. Granulocytes and monocytes from both WT and NLRX1 KO mice show increased phagocytic activity responses when challenged with E. coli at 37°C compared to 0°C (Figure 4D and E). However, no differences were observed in the percentage of granulocytes and monocytes that undergo phagocytosis between WT and NLRX1 KO (Figure 4E and F). Together, these results suggest that the early decreased bacterial clearance in the bladders from NLRX1 KO mice cannot be explained by an impaired granulocyte or monocyte response.
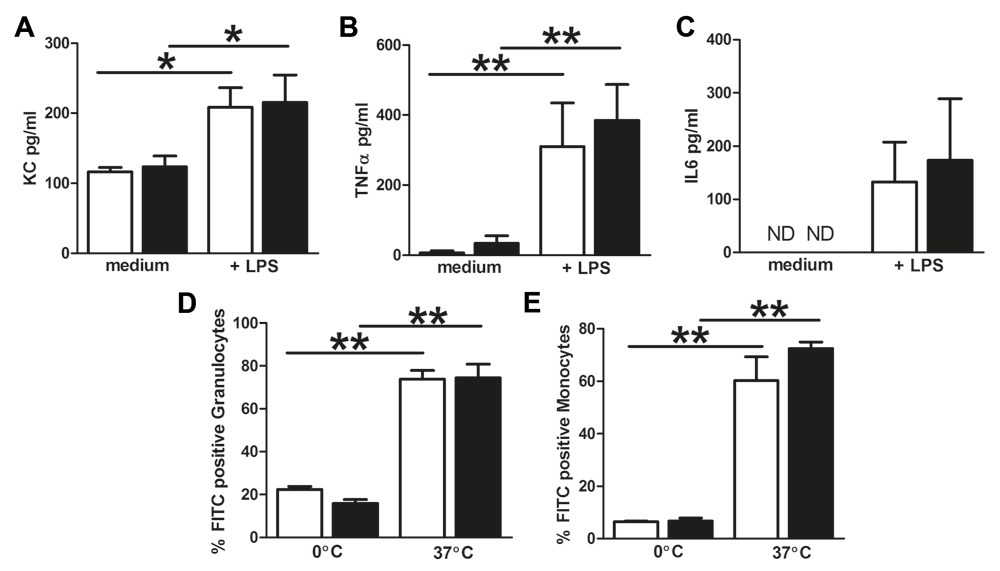
Levels of (A) KC (B) TNFα and (C) IL6 after 14h ex vivo LPS whole blood stimulation from wild-type (WT) (white bars) and NLRX1 knock-out (KO) (black bars). ND = not detectable. Phagocytic activity responses of (D) granulocytes and (E) monocytes from WT (white bars) and NLRX1 KO (black bars) mice ex vivo challenged with E. coli at 0°C and 37°C. All data are expressed as mean ± SEM, n=5–6 animals per group. Statistical significance between all columns was determined by non-parametric Mann Whitney U test, *P<0.05 and **P<0.01.
Innate immune receptors like TLRs and NLRs are known to play pivotal roles in the first line of host defense against invading pathogens. NLRX1 is an innate immune receptor that can modulate inflammatory responses23 and cell metabolism9,10. As such NLRX1 could play a potential role during the pathophysiology of UTI. To study this we investigated the role of NLRX1 during uropathogenic E. coli-induced lower and upper UTI in mice. Although NLRX1 enhances the inflammatory cytokine response and the bacterial clearance in the bladder during early experimental UTI, we found that this receptor does not affect overall renal bacterial loads and inflammation during pyelonephritis. In addition, we observed that NLRX1 is not essential for pro-inflammatory cytokine secretion by granulocytes and monocytes in response to LPS nor for phagocytosis of E. coli.
In this study we investigated the role of NLRX1 in influencing bacterial burden and inflammation in bladder and kidney during experimental UTI. Upon infection superficial cell exfoliation and inflammatory cell recruitment and activation, are key events in the complex host-pathogen interactions that take place in the bladder24. We found that the lack of NLRX1 is associated with an increased bacterial bladder burden at 24h. Studies by us (Supplementary Figure 325) and others26 show that the peak in uropathogenic E. coli outgrowth from C57BL6 mice bladders is observed before 24h. This, together with our observations, indicates that the bacterial clearance in the NLRX1 deficient bladder is delayed compared to WT. The impaired pro-inflammatory MIP-2 cytokine response which is usually needed for the recruitment of granulocytes to the site of infection22,27 and the initiation of host defense during UTI28 plays probably an important role in this. Surprisingly however, the levels of bladder MPO as an indicator of neutrophil influx and the ex vivo granulocyte phagocytic capacity to ingest E. coli are not affected by NLRX1 while local Nlrx1 expression in the bladder tended to be increased at 24h. Possibly, granulocyte influx is altered by NLRX1 at an earlier time point than 24h. It is in addition possible that processes like delayed E. coli-attachment, invasion and modulated exfoliation or factor secretion of superficial bladder cells contribute to the increased presence of E. coli bacteria in NLRX1 KO bladders at 24h. Whether or not direct or indirect NLRX1-mediated modulation of bladder cells contribute to the bacterial burden has not been proven yet and warrants further study. Despite the impaired ability to clear uropathogenic E. coli from the bladder at 24h and an impaired MIP-2, MCP-1 and TNFα cytokine response at 48h, NLRX1 KO are able to clear E. coli bacteria since the bacterial outgrowth in the bladder at 48h is not different in NLRX1 deficient mice compared to WT. Whereas in the kidney, the outgrowth from 24h to 48h in WT and NLRX1 KO remains unchanged, indicating that despite the local Nlrx1 expression increase at 48h NLRX1 does not affect bacterial outgrowth in the kidney. Our study demonstrates that due to NLRX1 absence, the MIP-2 cytokine release to recruit neutrophils is less pronounced and hence possibly attenuates the early phase of the host defense against E. coli in the bladder without affecting later bacterial bladder burden, innate myeloid cell phagocytosis and the promotion of pyelonephritis.
NLRX1 is on the one hand described to negatively regulate NF-κB signaling12,13 and on the other hand to indirectly amplify the NF-κB pathway29. During E. coli-induced UTI infections, the NF-κB signaling pathway is via TLR4 activation in parenchymal and bone-marrow derived cells crucial for the pro-inflammatory cytokine release and the clearance of E. coli from the urinary tract30 and31. In particular IL-6 is a major TLR-4 induced urinary cytokine that is released early upon E. coli bladder inoculation in mice and correlates with bacterial counts32. We observed in NLRX1 KO bladders, despite the increased bacterial counts at 24h post infection, that the levels of the pro-inflammatory cytokines TNFα, IL6 and IL1β were equal in both mouse strains. This indicates that upon early UTI, NLRX1 absence leads to a suppressed pro-inflammatory cytokine response in the bladder. Whereas at the later 48h time point in the bladder and at 24h and 48h in the kidney, NLRX1 has no effect on pro-inflammatory cytokine response and bacterial burden. Whether the suppressed pro-inflammatory cytokine response is caused by an altered neutrophil influx in the early onset of infection or the ability of NLRX1 to influence NF-κB signaling warrants further study. From our study it is however clear that NLRX1 is neither essential for whole blood pro-inflammatory KC, TNFα and IL6 cytokine responses to LPS, nor for bacterial phagocytosis by granulocytes and monocytes. Similar observations were done in bone marrow-derived macrophages where TNFα and IL6 cytokine expression remained similar in WT and NLRX1 deficient cells after a Helicobacter pylori (LPS positive) infection33. In contrast, TNFα and IL6 levels were increased upon NLRX1 knockdown in LPS-stimulated peritoneal macrophages12 and IL6 levels in LPS-stimulated bone marrow-derived macrophages13 indicating that NLRX1 attenuates NF-κB signaling. In contrast, increased NF-κB signaling upon LPS positive Shigella flexineri infection was observed in NLRX1 overexpressing epithelial cells29. Possibly, the role of NLRX1 varies between cell types involved in host defense, such as myeloid cells and parenchymal cells, during different time points of infection and different ligands. Together, we observed that NLRX1 does not affect the pro-inflammatory cytokine response after LPS challenge in granulocytes and monocytes, whereas previous studies show that in macrophages and epithelial cells NLRX1 can behave differently12,13,29. Indeed, during UTI epithelial cells are important in activating inflammation via various signaling pathways31. Based on our results that granulocyte and macrophage functioning are not affected by NLRX1, we assume that during UTI NLRX1 plays a role in the early bacterial burden in the bladder by activating the pro-inflammatory cytokine response in urinary tract parenchymal cells via NF-κB.
Besides its role in immune regulation, we previously observed that NLRX1 functioning extends to the control of mitochondrial activity, oxidative stress and cellular metabolism in parenchymal cells of the kidney and liver9,10. Macrophages and in particular neutrophils contribute during infections to the host defense via oxidative burst34,35. From our data it is not clear if NLRX1 plays a role in the oxidative burst in myeloid cells during UTI. However, a previous study showed that NLRX1 plays no significant role in ROS production of LPS activated neutrophils and macrophages13. Together, our data indicates that there is a role for NLRX1 during UTI in the bladder by activating the pro-inflammatory cytokine response, while no direct role for NLRX1 is observed in myeloid cells.
We report that NLRX1 plays a role in attenuating the early uropathogenic E. coli bacterial burden in the bladder however this has no consequences for the development of bacterial burden in the bladder at a later time point nor for the development of pyelonephritis.
Dataset 1: NLRX1 expression data – This file contains the data underlying the analysis of the NLRX1 expression in bladder and kidney as shown in Figure 1. https://doi.org/10.6084/m9.figshare.9879635.v136
Dataset 2: In vivo mice bladder and kidney colony forming units (CFU)-, cytokine-, general marker-data and leukocyte counts in sham and after 24h and 48h of infection – This file contains the data underlying the analysis of the data used in Figure 2, Figure 3 and Supplementary Figure 1. https://doi.org/10.6084/m9.figshare.9879632.v137
Dataset 3: Ex vivo cytokine data – This file contains the data underlying the cytokine determination in LPS stimulated whole blood as shown in Figure 4. https://doi.org/10.6084/m9.figshare.9879629.v138
Dataset 4: Ex vivo FACS output data - This file contains the FACS data underlying the leukocyte composition analysis as shown in Figure 3. https://doi.org/10.6084/m9.figshare.9879620.v139
Dataset 5: Ex vivo FACS output data on granuocyte and monocytes phagocytosis - This file contains the FACS data underlying the granuocytes and monocytes phagocytosis as shown in Figure 4. https://doi.org/10.6084/m9.figshare.9879611.v140
Dataset 6: In vivo mice bladder colony forming units (CFU) data of WT mice 4h, 8h, 24h and 48h of infection – This file contains the data underlying the analysis of the data used in Supplementary Figure 325. https://doi.org/10.6084/m9.figshare.9879455.v141
Supplementary Figure 1. Renal function of wild-type (WT) and NLRX1 knock-out (KO) mice during experimental urinary tract infections (UTI).
Plasma levels of renal function markers (A) urea and (B) creatinine from sham, 24h and 48h uropathogenic Escherichia coli inoculated WT (white bars) and NLRX1 KO (black bars) mice. All data are expressed as mean ± SEM, n=3–4 (sham) and n=7–8 (24h and 48h) animals per group. Statistical significance was determined by non-parametric Mann Whitney U test.
https://doi.org/10.6084/m9.figshare.9879662.v121
Supplementary Figure 2. Gating strategy of circulating leukocytes.
Of total leukocytes, living cells, granulocyte (population 1), monocyte (population 2) and lymphocyte (population 3) populations were determined based on forward-scattered light (FSC)/side-scattered light (SCC). For the phagocytosis capacity experiments FSC/FITC gates were set based on the negative control and Escherichia coli FITC positive cells were identified in population 1 and population 2.
https://doi.org/10.6084/m9.figshare.9879677.v118
Supplementary Figure 3. Bacterial outgrowth bladder.
Outgrowth of uropathogenic E. coli expressed in colony forming units (CFU) in bladder homogenates from wild-type (WT) mice 4h, 8h, 24h and 48h after inoculation. Detection limit: 10 CFU/ml.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: urinary tract infection, Escherichia coli, bacterial pathogenesis
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: urinary tract infection, Escherichia coli, bacterial pathogenesis
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: urinary tract infection, Escherichia coli, bacterial pathogenesis
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 24 Sep 19 |
read | |
|
Version 2 (revision) 11 Apr 19 |
read | |
|
Version 1 06 Aug 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)