Keywords
RNA, NMD, evolution, UPF1, SMG1, transposable element, RNA decay
RNA, NMD, evolution, UPF1, SMG1, transposable element, RNA decay
Gene expression is controlled by a variety of mechanisms, sometimes in unexpected ways. Early mutant screens identified mutations that introduced nonsense mutations, but surprisingly, these premature termination codons (PTCs) lead to a reduction in mRNA stability1,2. This increase in RNA decay is the result of an active translation-dependent process1,3. This pathway was termed nonsense-mediated mRNA decay (NMD) and is now known to regulate hundreds to thousands of transcripts in plants, animals and fungi4–8. Many of the NMD targeted transcripts are not the result of nonsense mutations, but are instead the result of alternative splicing events that introduce PTCs or the presence of an upstream open reading frame (uORF). Many such splicing events are not the result of splicing errors, but are in fact highly conserved events9,10. Therefore, NMD has a major role in shaping the transcriptome of diverse eukaryotes. However, the exact molecular nature of the NMD pathway varies between organisms. Most eukaryotes share the core NMD factors (see below), but an impressive number of modifications to the NMD pathway exist. In this review, I will examine the factors known to act in NMD, discuss the diversity of these factors in eukaryotes, and explore the different mechanisms that explain how a PTC is differentiated from an authentic stop codon. Finally, I will discuss how the NMD pathway may have evolved and some remaining key questions in our understanding of the NMD pathway.
Early mutant screens in baker's yeast and Caenorhabditis elegans identified three conserved factors that could suppress a nonsense mutation11,12. These factors were named UP-frameshift (UPF) 1, 2 and 3 in baker's yeast and suppressors with morphological defects on genitalia (SMG) 2, 3 and 4 in C. elegans. The baker's yeast names of these factors are used throughout this review. UPF1 is a highly conserved RNA helicase13 that interacts with UPF2, which is an MIF4G domain-containing protein14, that in turn binds to UPF3 (Figure 1)15,16. The initial mutant screens in C. elegans also revealed four additional factors: the kinase SMG1 and the 14-3-3-like domain proteins SMG5, SMG6 and SMG711,17. In animals, SMG1 is known to phosphorylate UPF1 after a PTC is been recognised (Figure 1)18–20. Initially, NMD factors were defined by their role in the phosphorylation of UPF1. UPF2 and UPF3 support the phosphorylation of UPF1 by creating a complex compatible for phosphorylation by SMG120, while also acting to activate the RNA helicase activity of UPF122. SMG5-7 bind to phosphorylated UPF123 and are active in the dephosphorylation of UPF1 by recruiting the PP2A phosphatase24–26. However, it is now clear that their primary role is in acting at various stages of RNA decay. SMG5-7 have a central role in recruiting the degradation machinery to degrade the NMD target (Figure 1). SMG5 and SMG7 act to recruit exonucleases27, while SMG6 is an endonuclease, cutting the transcript near the PTC28,29. Over time, many more NMD factors have been identified through further genetic and biochemical screens30–33. Of these, SMG8 and SMG9 are of particular interest. First identified in human cells as SMG1-interacting proteins, they act in the NMD pathway of humans and possibly C. elegans32,34 through the inhibition of the kinase SMG1. Curiously, studies in mammals have revealed that many NMD targets do not require the involvement of all NMD factors. Many NMD targets use “branches” of the NMD pathway that do not require UPF235 or UPF3b36. However, all branches do involve UPF1, highlightings its central importance to the NMD pathway.
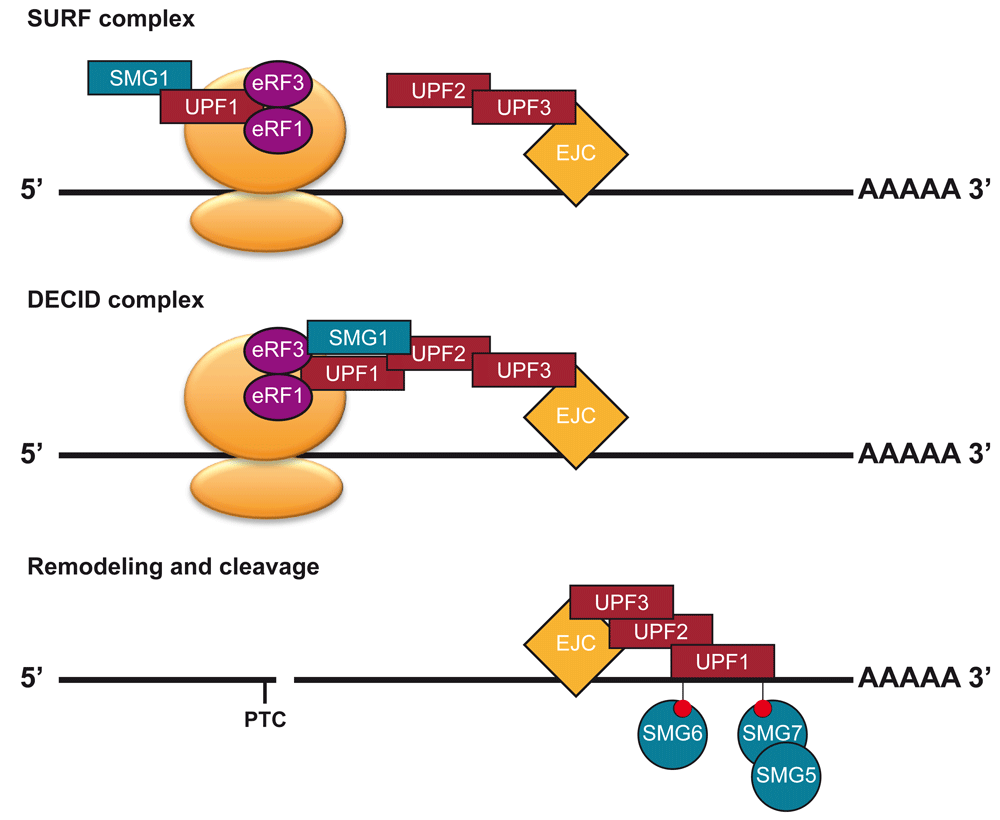
At termination events, UPF1 and SMG1 are recruited to termination events by eRF1 and eRF3, leading to the formation of the SMG1-Upf1-eRF1-eRF3 (SURF) complex20. If an EJC, bound by UPF3 and UPF2, is present downstream of the terminating ribosome, then the decay-inducing (DECID) complex will form20,21. This will lead to the phosphorylation of UPF1 by SMG1. Then the ribosome will disassociate and SMG5-7 will be recruited to transcript through phoso-UPF1 binding. The transcript is degraded by nucleases.
Together these studies, mostly using animal systems, paint a picture where multiple factors (UPF2, UPF3, SMG1, SMG8, and SMG9) assist in the activation of UPF1, while other factors (SMG5-7) act to degrade an NMD target and dephosphorylate UPF1.
Despite the deduction of a basic schematic of the NMD pathway in animals (Figure 1), many of the factors involved in this classical model of NMD vary between different organisms (Figure 2 and Figure 3). The most highly divergent NMD pathways are those found in the excavata (Figure 2 and Figure 3). The excavata have been suggested to be the most basal group of eukaryotes37, although other work places them within the same supergroup as plants38,39. Although the NMD pathways of the parasites Giardia lamblia and Trypanosoma brucei have been studied, it is unclear if a functional NMD pathway exists in these organisms40,41. They contain heavily reduced compliments of NMD factors: the genome of G. lamblia only harbors UPF1, and the genome of T. brucei only harbors UPF1 and UPF240,41. Over-expression of UPF1 in G. lamblia caused an NMD reporter to further decrease, suggesting that G. lamblia might have an active NMD pathway40. In contrast, the knockdown of UPF1 in T. brucei did not increase NMD reporter construct expression, or endogenous genes41. However, tethering of UPF1 in T. brucei did decrease reporter expression41. Therefore, it is difficult to definitively conclude the status of the NMD pathway in excavata. However, it is worth noting that parasites are known to have reduced genomes relative to free-living relatives42, and that the excavata Naegleria gruberi does harbor the additional NMD factors of SMG1 and SMG943. This indicates that a complex NMD pathway involving the kinase SMG1 likely existed in the last eukaryotic common ancestor.
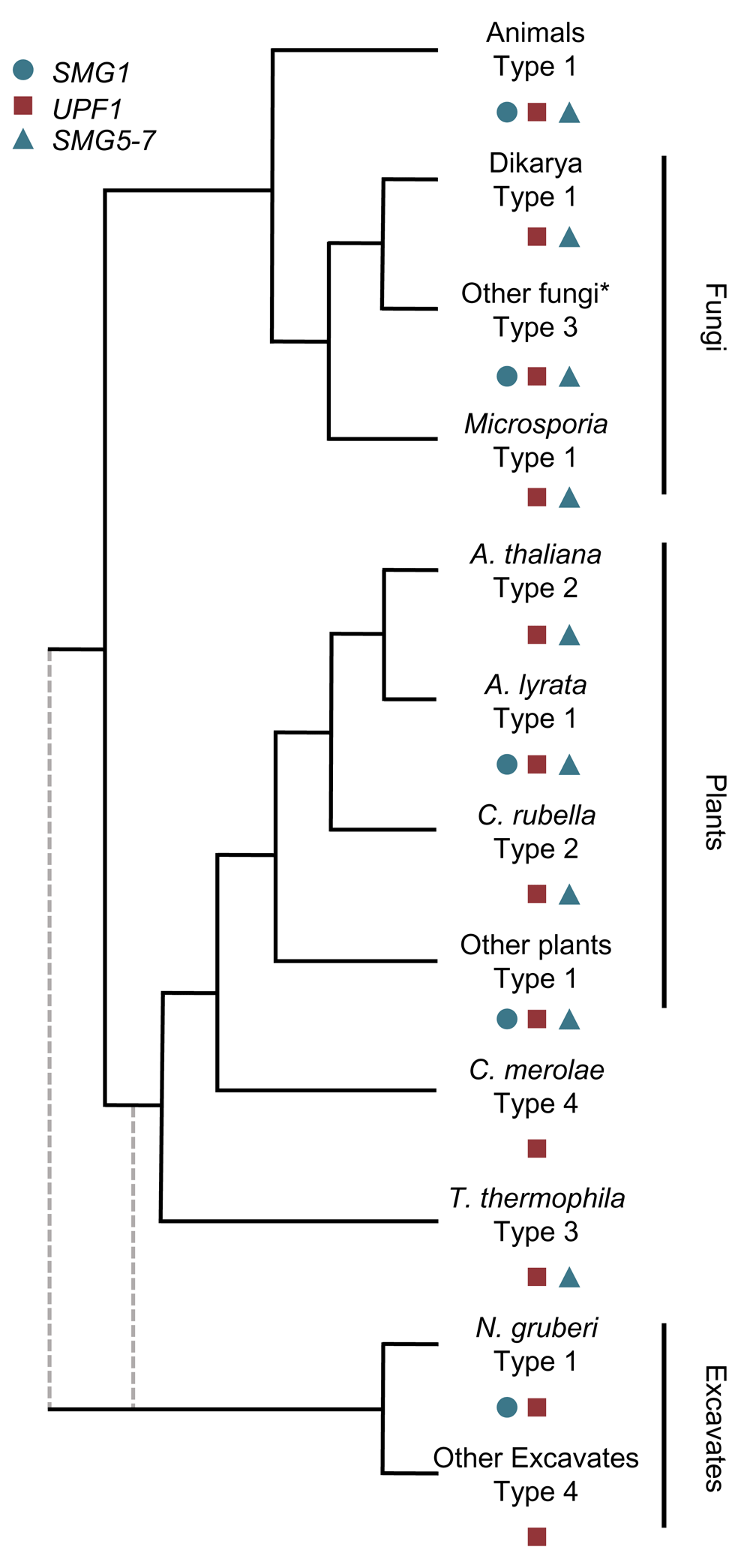
The distribution of the key NMD factors, UPF1, SMG1 and a member of the SMG5-7 family define the NMD pathway type. All NMD types have arisen multiple times within eukaryotic evolution. To date, no SMG5-7 family member has yet been identified in Naegleria gruberi but given the presence of SMG143, I am currently classifying it as a type 1 NMD pathway. The branch lengths do not reflect the relatedness of any species, but represent the order of separation between the lineages. The root of eukaryotes is unclear, so branches representing a Excavata early and late divergence are represent in grey, dashed-lines. *SMG1 appears to have been lost in other fungal lineages as well, representing repeated losses in multiple fungal lineages43. NMD pathways can be classified into four types (Figure 3).
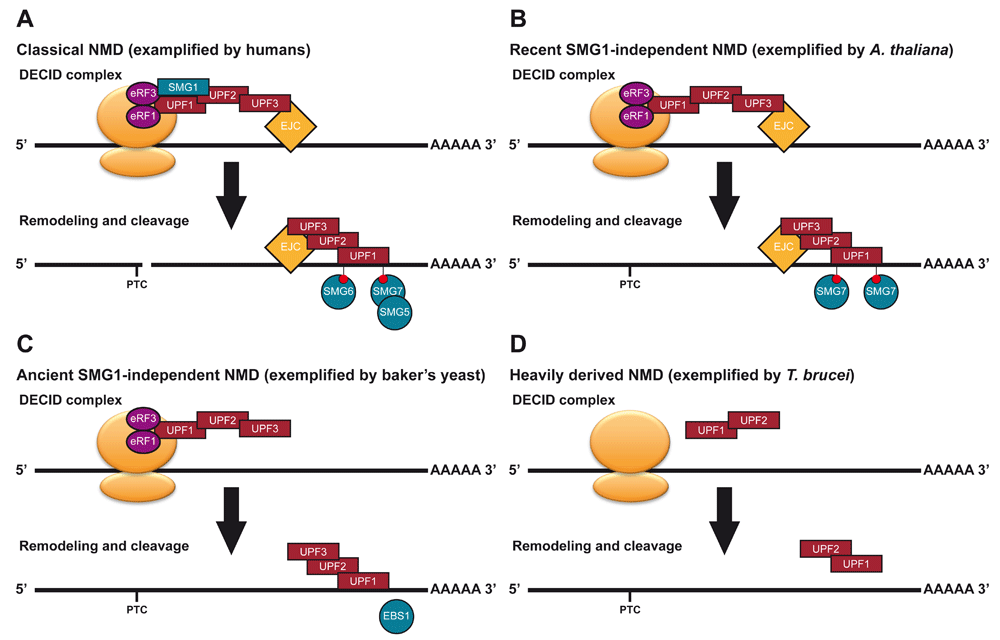
(A) Classical NMD, exemplified by humans (modified from Figure 1). (B) Recent SMG1-independent NMD, exemplified by A. thaliana. A. thaliana lost SMG1 within the last 5–10 million years43,44. A. thaliana requires SMG7 for a functional NMD pathway45, retains a S/TQ rich UPF143 and its UPF1 needs to be phosphorylated to function in NMD in tobacco leaves46,47. This suggests an alternative kinase may have replaced SMG1. (C) Ancient SMG1-independent NMD, exemplified by baker’s yeast. The NMD pathway of baker’s yeast was the first to be characterised. UPF1, UPF2 and UPF3 have central roles in this pathway. Reverse genetics revealed a potential lesser role for EBS1, a SMG7 homologue, in NMD48 but its UPF1 is depleted in S/TQ dipeptides43. (D) Heavily derived NMD, exemplified by T. brucei. It is unclear if a functional NMD pathway exists in these organisms. In T, it has been shown that UPF1 and UPF2 interact, but their interaction with the ribosome and potential NMD targets is unclear41. Tethering of UPF1 a transcript can decrease its abundance41.
Further support for this comes from the examination of plant NMD pathways. Plants, which diverged from animals and fungi early in eukaryotic evolution (Figure 2), do have functional homologues of the NMD holy trinity: UPF1-349,50. Plants also have homologues of SMG5-7, known as SMG7 and SMG7-like45, and SMG1 homologues43,44. SMG1 has been repeatedly lost throughout eukaryotic evolution, including two losses in land plants (Arabidopsis thaliana and Capsella rubella) and multiple losses in fungi (Figure 2)43,44. The repeated loss of SMG1 raises some interesting questions about the mechanism of NMD activation. In animals, and presumably in most plants, SMG1 phosphorylates SQ and TQ dipeptides at the N- and C-termini of UPF118,46,51. Species, such as baker’s yeast, with an ancient loss of SMG1 (Figure 2), have UPF1 sequences depleted of S/TQ dipeptides relative to species with SMG143. Species that lost SMG1 more recently, such as A. thaliana, have UPF1 proteins that are rich in S/TQ dipeptides43. The repeated losses of SMG1 in eukaryotes suggests that there is a genetic buffer, another factor/mechanisms that allows SMG1 to be lost but the NMD pathway to be activated43,44. In support of this notion, the experimental perturbation of SMG1 in fruit flies and zebrafish has little or no effect on the NMD pathway of these organisms52–54, suggesting that a backup UPF1-activation mechanism is already present in these species. Alternatively, different mechanisms have replaced SMG1 in each independent loss of SMG1, which might explain why some organisms have retained S/TQ richness within their UPF1 protein sequences while others have not43,44.
The SMG5-7 family split and diversified in the animal lineage, with the acquisition of the PIN domain in SMG5 and SMG626,55,56. The PIN domain of SMG6 gives it the ability to act as an endonuclease, cutting the NMD targeted transcript near the PTC28,29. The SMG5-7 family also have a role in regulating telomere length57. SMG5-7 homologues in plants are known as SMG7, given they lack the PIN domain of SMG5 and SMG645. SMG5-7 family members of baker’s yeast, EBS1 and ETS1, also lack the PIN domain48. In baker’s yeast, ETS1 is implicated in telomere regulation but not NMD, while a knockout of EBS1 reveals a mild NMD phenotype48. Given that baker’s yeast lacks SMG119,43,44, it is not clear why EBS1/SMG7 would be required for NMD. The UPF1 of baker’s yeast is depleted of S/TQ dipeptides43, which once phosphorylated by SMG1, normally act as binding site for SMG5-723. The lack of S/TQ dipeptides suggest that classical phosphorylation of UPF1 is not required for the activation of NMD in baker’s yeast. Tyrosine phosphorylation of UPF1 in baker’s yeast has been observed and appears to regulate the RNA helicase activity of UPF158. It is possible that these or other phosphorylated sites could act to recruit decay factors like S/TQ dipeptides do. However, given the differences between S and T residues from Y, it seems unlikely that EBS1/SMG7 would be involved. Recently, another member of the SMG5-7 family was characterized in the ciliate Tetrahymena thermophila59, despite the loss of the SMG1 kinase from T. thermophila43,59. The SMG5-7 family member of T. thermophila was named SMG6-like (SMG6L) due to the presence of the C-terminal NYN nuclease domain, potentially taking on the same role as the PIN domain of animal SMG6 proteins59. SMG6L appears to work with UPF1 in the NMD pathway of T. thermophila and is conserved in many other protozoa59. However, it is unclear how it is recruited to UPF1 given the lack of SMG1 and classical phosphorylation sites on UPF1.
The kinase activity of SMG1 is regulated in part by SMG8 and SMG932. These factors have been identified but not characterized outside of the animal kingdom43; a curious finding which indicates they may have a role in NMD in diverse eukaryotes. When SMG1 is lost from a genome, SMG8 and SMG9 are generally also lost43. Further work will be needed to reveal the extent of any conserved role in NMD for these factors.
Taken together, a diverse set of NMD pathways with varying levels of classically defined NMD factors been identified. Generally speaking, these can be split into four major types (Figure 2 and Figure 3):
1) Classical SMG1-dependent NMD (As exemplified by C. elegans, humans, and moss)
2) Recent SMG1-independent NMD (As exemplified by A. thaliana)
3) Ancient SMG1-independent NMD (As exemplified by baker’s yeast and T. thermophila)
4) Heavily derived NMD (As exemplified by G. lamblia, T. brucei and Cyanidioschyzon merolae)
Type 1 NMD pathways (classical SMG1-dependent NMD; Figure 3A) are known to exist in both animals and plants18,19,44 and is likely to be the ancestral state of NMD43,44. However, even here, the dependence on SMG1 is not always clear: SMG1 mutants in fruit flies have much milder phenotypes than mutations in other NMD factors53,60 and knockdown of SMG1 in zebrafish revealed no phenotype54. It is possible that the NMD pathways of some species with a type 1 NMD pathway in appearance might better resemble type 2 NMD (Recent SMG1-independent NMD).
Type 2 NMD pathways (recent SMG1-independent NMD; Figure 3B), such as those of the land plants A. thaliana and C. rubella, appear very much like those of type 1, with the exception of SMG1 being absent from the genome, likely with the accompanying loss of SMG8 and SMG943,44. However, UPF1 still maintains the relatively high level of S/TQ dipeptide phosphorylation sites43, and phospho-UPF1 binding protein SMG78,45. It would be tempting to speculate that a kinase related to SMG1 replaced it in the NMD pathway44. ATM and ATR are two kinases from the same family as SMG1 that are conserved in plants and are involved in DNA repair. However, in A. thaliana, the reported mutant phenotypes of ATM and ATR61 do not overlap with the classical NMD phenotypes49, so this seems unlikely to be the case.
A type 3 NMD pathway (ancient SMG1-independent NMD; Figure 3C), was the first to be characterized by a mutant screen in baker’s yeast12,62. These ancient losses of SMG1 lead to an NMD pathway without SMG1, without SMG8 and SMG943, with UPF1 depleted in S/TQ dipeptides43, and an unclear role for SMG5-7 proteins48,59. Future work (see below) will be needed to better understand the exact molecular role of SMG5-7 proteins in type 3 NMD pathways, and to understand how the NMD pathway functions without the SMG1 activating UPF1.
Type 4 NMD pathways (heavily derived NMD; Figure 3D) are the most variable group and are found throughout the eukaryotic tree of life. These pathways often lack SMG1, but also core NMD factors (UPF2 and UPF3). Although UPF3 is hard to identify with homology searches63, it does appear to be missing from the genomes of a number of species43. These include the excavata parasites G. lamblia and T. brucei40,41 but also the red algae C. merolae44. C. merolae has a very reduced genome, with only 27 introns in total64. C. merolae and G. lamblia also lack homologues of UPF2. It is certainly possible that the presence of these factors do not represent a fully functional form of an NMD pathway and instead reflect the molecular reminance of a former NMD pathway whose factors have not been co-opted for other functions.
In any of these species, additional NMD factors are likely to have arisen. PNRC2 is a vertebrate-specific NMD factor33. The only non-type 1 species to have had a forward genetics screen performed for is the baker’s yeast, so we have limited unbiased studies to draw from. More forward screens and biochemical studies are likely to yield more species-specific factors. This will be especially exciting in type 4 species, with the most heavily reduced NMD pathways. This framework of NMD types based on presence/absence of conserved NMD factor is aimed at aiding the comparison and discussion of NMD pathways from diverse organisms. Thinking of all NMD pathways as being fundamentally the same at the molecular level is wrong. There is certainly an overlap, but more focused studies are needed to understand when homologous NMD factors do have the same molecular role in NMD and do not.
So far I have discussed the molecular processes that link the recognition of a PTC to transcript destruction. However, a lot of work has also been focused on understanding the mechanism of how a PTC is differentiated from an authentic stop codon. Multiple models for how this is achieved have been proposed. One of the most well characterized models centres around the exon junction complex (EJC), a protein complex deposited on an mRNA after two exons are ligated together during splicing65,66. While most EJCs are removed from the transcript by the translating ribosome67, EJCs associated with exon-exon junctions ≥50 nt downstream of a stop codon are not removed and can elicit NMD68,69. Early work showed that the EJC was not involved in the NMD pathways of fruit flies56, but more recent work proved the contrary, revealing a role for the EJC in fruit fly NMD70. The EJC has been lost from baker’s yeast and so cannot have a role in its NMD pathway, but the EJC is involved in the fungi Neurospora crassa’s NMD pathway71. The EJC mode has even found support in plants, with reporter genes and transcriptome-wide studies supporting a role for exon-exon junctions in 3’ UTRs eliciting NMD50,72–74. These findings would suggest that the EJC mode is an ancient mechanism for targeting transcripts to NMD. A surprising version of the EJC mode is the finding that NMD in T. thermophila is dependent on splice junctions downstream of the stop codon, but not on the EJC itself59. Knockout of the core EJC component Mago nashi did not alter the expression levels of NMD targets identified by knockout of UPF1 and SMG6L59. This indicates that an alternative mechanism might maintain an EJC-like mode of NMD in T. thermophila.
Another well-explored system used in defining PTCs is the long 3’ UTR mode. Transcripts with abnormally long 3’ UTRs have been found in reporter genes50,72,75 and transcriptome-wide studies5,73,76 to target transcripts to NMD, although some recent transcriptome-wide studies found little to no trend across the transcriptome, when the presence of 3’ UTR introns were taken into account59,74,77,78. One proposed mechanism is the increased distance between the stop codon (PTC) and the polyA-binding protein, bound to the polyA tail79,80. This physical separation between the polyA tail and the terminating ribosome might lead to aberrant termination and the recruitment of NMD factors79,80. An alternative model posits that longer 3’ UTRs are able to recruit more UPF1 directly bound to the 3’ UTR81. It has been found that UPF1 coats transcripts, but translation displaces UPF1 from all regions, except the 3’ UTRs82. This model suggests that a higher level of UPF1 binding increases the chances of NMD being triggered during the termination of translation; Naturally long 3’ UTRs that are resistant to NMD have been observed to bind less UPF1 than susceptible long 3’ UTR transcripts81. In fact, some naturally long 3’ UTR transcripts appear to be protected from NMD by various features such as a recently identified cis-sequence element in the TRAM1 gene83 or the many genes found to bind PTBP1 near the stop codon to prevent NMD84. Such features protecting long 3’ UTR transcripts from NMD might explain why transcriptome-wide studies find so few long 3’ UTR transcripts that are targeted to NMD.
In baker’s yeast, a downstream sequence element (DSE) was identified85,86. When this sequence is downstream of a stop codon, NMD is elicited, likely through the recruitment of an RNA binding protein85,86. This mechanism is very similar to the way in which the EJC mode works in animals and plants. The existence of DSEs in other species are possible, but to date, none have been identified. Fission yeast also display an unusual PTC recognition mechanism, not yet observed in other species: splicing near a stop codon, either upstream or downstream of the stop codon, can induce NMD and is independent of the EJC87. Whether such a mechanism exists in other eukaryotes and what factors determine this remain to be seen.
The mechanisms used to define PTCs in the last eukaryotic common ancestor are unclear. While the EJC mode has been identified in both plants and animals, suggesting an ancient origin, there are many eukaryotic lineages where it has not been characterized, or does not function59,87. The long 3’ UTR mode of NMD has also been characterized in many diverse eukaryotes (plants, animals and fungi), but the mechanism underlying this mode, and failure to observe a strong signal for this feature in transcriptome-wide studies, does raise questions.
Today, eukaryotes appear to utilize NMD in a variety of ways to achieve the same aim, degrading PTC-containing transcripts from a variety of sources. It is clear that a rather complex NMD pathway, belonging to the type 1 group, existed in the last eukaryotic common ancestor (see above). In extant diploid eukaryotes, NMD can prevent some mutations from being dominant, protecting heterozygous individuals by turning these alleles recessive11,88,89. However, NMD also increases the severity of some genetic disorders90, creating a double-edged sword: protecting some mutation-carrying individuals while exacerbating the conditions of others. Therefore, it is unlikely that protecting the genome from nonsense mutations was the driving force behind the origin of the NMD pathway. Early eukaryotes did face a particular selective pressure not present in prokaryotes: rapidly multiplying transposable elements (TEs). The origin of sex in eukaryotes allowed for TEs to expand in copy number, which is not possible in prokaryotes with their primarily asexual reproductive system91,92. With the advent of sex, eukaryotes faced the expansion of many TE classes, including the self-splicing (group II) introns. Expansion of group II introns has been proposed to have driven the evolution of the spliceosome to enhance the splicing of these selfish elements93, the nucleus evolved to physically separate the processes of transcription and translation and allow for intron removal before translation94, and NMD evolved to degrade intron-retaining transcripts that escaped the nucleus94. These adaptations ensure that retention of these efficiently-spliced introns would not be repeatedly translation. More recent expansions of introns in some eukaryotic lineages are due to the expansion of DNA transposons95, indicating the importance of these mechanisms to protect the genome from TE expansions in extant eukaryotes, and suggests multiple origins for introns from TEs throughout eukaryotic evolution. Once functional as a TE-intron protection pathway, NMD appears to have been co-opted to control gene expression. Today, in addition to repressing the expression of uORF-containing genes, pseudogenes and the products of alternative splicing, NMD may allow for the evolution of new introns. The presence of NMD may act as a buffer for novel introns with weak splice sites96. In fact, the red algae C. merolae only has 27 introns64 and is missing all of the classical NMD factors with the exception of a UPF1 homologue44. It is possible that C. merolae lacks a functional NMD pathway and this limits the acquisition of new introns, at least partly explaining its intron depleted genome.
Many years of study have revealed diverse NMD pathways, centering on UPF1. However, there are a number of fundamental questions remaining in the field regarding the mechanisms and evolution of NMD.
1) Why is SMG1 repeatedly lost in different lineages? Is there a backup mechanism to activate UPF1 and is this conserved between the lineages that have recently lost (eg A. thaliana) and more anciently (eg baker’s yeast and T. thermophila) SMG1? Or are there multiple SMG1 replacement mechanisms?
2) What recruits the RNA degradation machinery to UPF1 when SMG1 is lost and S/TQ dipeptides are depleted? Does this still depend on the SMG5-7 family and UPF1 phosphorylation?
3) What precisely are the roles of SMG5-7 family members in lineages that have lost SMG1? Do their roles differ between species with type 2 (recent loss of SMG1) and type 3 (ancient loss of SMG1) NMD pathways?
4) What is the molecular basis of an EJC mode of PTC recognition when the EJC is not involved, such as in T. thermophila?
5) What is the precise mechanistic roles of UPF2/UPF3 in relation to EJC mode and non-EJC mode NMD pathways?
6) To understand the discrepancies between transcriptome-wide and reporter construct approaches to the long 3’ UTR mode of NMD and to uncover the molecular mechanism(s) behind the long 3’ UTR mode.
Hopefully future research efforts can resolve these and other unknowns surrounding NMD.
Here I have discussed the NMD pathway in the context of evolution and the many shapes the NMD pathways takes. I have proposed a classification system with four types of NMD pathway, based on the presence/absence of conserved NMD factors. I propose that the classical (type 1) NMD involves UPF1-3, the UPF1-kinase SMG1 and the SMG5-7 family. The recent (type 2) and ancient (type 3) loss of SMG1 define the next two types of NMD, while loss of all but UPF1 and perhaps UPF2 define the final type (type 4), where NMD might not actually function at all. It is highly likely that species specific NMD factors have been co-opted in many, if not all, of these types of NMD pathway and are waiting to be discovered. Discussing the evolution and mechanism of NMD within this framework will hopefully aid in the communication of ideas between different model systems used to study NMD and therefore help in knowledge acquisition. Finally, I outline key outstanding questions regarding the mechanism and evolution of the NMD pathway. Focused research efforts to address these issues will certainly help in our overall understanding of the NMD pathway and for us to at last appreciate the true fundamental nature of NMD.
No data are associated with this article.
This work was supported by the Australian Research Council (ARC) Centre of Excellence program in Plant Energy Biology CE140100008.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Thanks to Barry Causier, Suruchi Roychoudhry and Joanna Franklin-Lloyd for critical feedback on this review. Thanks to the Centre of Excellence in Plant Energy Biology, Australian Research Council (CE140100008) for funding.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Swisher KD, Parker R: Interactions between Upf1 and the decapping factors Edc3 and Pat1 in Saccharomyces cerevisiae.PLoS One. 2011; 6 (10): e26547 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Reichenbach P, Höss M, Azzalin C, Nabholz M, et al.: A Human Homolog of Yeast Est1 Associates with Telomerase and Uncaps Chromosome Ends When Overexpressed. Current Biology. 2003; 13 (7): 568-574 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: mRNA turnover (nonsense-mediated mRNA decay)
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Ruiz-Echevarría MJ, Peltz SW: The RNA binding protein Pub1 modulates the stability of transcripts containing upstream open reading frames.Cell. 2000; 101 (7): 741-51 PubMed AbstractCompeting Interests: No competing interests were disclosed.
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Lareau LF, Brenner SE: Regulation of splicing factors by alternative splicing and NMD is conserved between kingdoms yet evolutionarily flexible.Mol Biol Evol. 2015; 32 (4): 1072-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 22 Nov 18 |
||||
|
Version 1 15 Aug 18 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)