Keywords
Malate dehydrogenase, Saccharomyces cerevisiae, antibody, western blot
This article is included in the Antibody Validations gateway.
Malate dehydrogenase, Saccharomyces cerevisiae, antibody, western blot
Malate dehydrogenases (Mdhs) catalyse the interconversion of malate and oxaloacetate using NAD+ or NADH1. In the yeast Saccharomyces cerevisiae there are three known isozymes: Mdh1 is located in mitochondria, Mdh2 is mostly cytosolic, and Mdh3 is localized to peroxisomes. The residues of the three isozymes are between 43–50% identical2. It is thus very important for purposes of isolation and identification to utilize specific antibodies that will recognize only one specific isozyme. Using three Mdh2 peptides, which were specifically designed to unique regions in the Mdh2 protein, GenScript USA produced three different antibodies that should have high specificity for S. cerevisiae Mdh2 relative to Mdh1 and Mdh3. We then tested all three antibodies by western blotting and found one with specific binding. Due to its specificity, this antibody has the potential to also work in other experimental assays such as immunoprecipitation, immunohistochemistry and ELISA (Enzyme-Linked Immunosorbent Assay).
Three antibodies for S. cerevisae Mdh2 were custom produced for us by GenScript USA : 1. Purified antibody, Anti-peptide #1, item number: U1684BK300_2, LOT number: A416120074, catalogue number: SC1195. 2. Purified antibody, Anti-peptide #2, item number: U1684BK300_5, LOT number: A416120094, catalogue number: SC1195. 3. Purified antibody, Anti-peptide #3, item number: U1684BK300_8, LOT number: A416120072, catalogue number: SC1195.
All three antibodies were raised in New Zealand Rabbit and are polyclonal. The immunogen for all three antibodies is conjugated KLH Peptide. To find specific Mdh2 peptides, the three S. cerevisiae malate dehydrogenases (Mdh1, Mdh2 and Mdh3) were aligned and analysed by GenScript, using their Antigen Design Tool. From this analysis, three peptides (corresponding to the three antibodies) were selected: #1: CHPQSRNSGIERRIM; #2: CINIESGLTPRVNSM; #3: MPHSVTPSIEQDSLC. The cysteines in the N’ terminus (peptides number 1 and 2) or C’ terminus (peptide number 3) were added for KLH conjugation.
Yeast strains are all based on the BY4741 laboratory strain3. Manipulations were performed using a standard PEG/LiAC transformation protocol4. The GFP-Mdh1, GFP-Mdh2, GFP-Mdh3 and OE-mCherry-Mdh3 strains were picked from the SWAT N’ GFP or N’ mCherry yeast libraries that were recently prepared in our lab5. All Strains with a fluorescent tag, including the strains that were picked from the SWAT libraries were verified using PCR (one primer from within the endogenous Open Reading Frame (ORF) and one from within the tag sequence) as well as by fluorescent microscopy. Primers for creating strains with deletions or C’ tagging of genes (Δmdh1, Δmdh2, Δmdh3 and Mdh2-mCherry) were designed using the Primers-4-Yeast web tool6. All deletions were verified using primers from within the endogenous ORF. All primers are summarized in Table 1.
Yeast proteins were extracted by the NaOH protocol as previously described7 and resolved on polyacrylamide gels with the following modifications:
1. Liquid cultures were grown over night at 30°C in glucose containing media, and then were diluted and incubated for an additional 4–6 hours to get to mid-log phase. Cells that were grown in a different carbon source than glucose were grown in synthetic media with 0.1% glucose (6.7 g/l yeast nitrogen base and 0.1% glucose) for the overnight incubation, then diluted in synthetic media containing galactose (6.7 g/l yeast nitrogen base and 2% galactose) or oleic acid (6.7 g/l yeast nitrogen base, 0.2% oleic acid and 0.1% Tween-80) and incubated for an additional 4–6 hours. Cells that were grown in glucose were grown in yeast extract peptone dextrose (YPD) rich medium (1% Yeast Extract, 2% peptone, 2% glucose) for the whole process.
2. The extractions were incubated for 10 minutes at 70°C and 15 µl supernatant was loaded per lane of the gel.
The gels were transferred to nitrocellulose membrane blots, blocked for 1 hour in SEA block diluted in TBS-T (1:5) at room temperature (RT), and probed with primary rabbit/mouse antibodies against Mdh2, Histone H3, Actin and mCherry (Table 2) for 1 hour at RT. Final concentrations of the primary antibodies were: 1 µg/ml for anti-Mdh2 peptides #1 and #3, 0.5 µg/ml for anti-Mdh2 peptide #2, anti-mCherry and anti-Actin and 0.2 µg/ml for anti-Histone H3. The membranes were washed 3 times without incubation in TBS-T, followed by 3x3 minute washes in TBS-T. The membranes were then probed with a secondary goat anti-rabbit/mouse antibody conjugated to IRDye800 or to IRDye680 (Table 2) for 30 minutes at RT and washed as before. Final concentrations of the secondary antibodies were 0.1 µg/ml for IRDye 800CW Goat anti-Rabbit IgG and IRDye 680RD Goat anti-mouse IgG, and 0.2 µg/ml for Goat anti-mouse HRP conjugated. Membranes were scanned for infrared signal using the Odyssey Imaging System (Li-Cor). For detecting the anti-Actin primary antibody (Table 2), a goat anti-mouse HRP conjugated secondary antibody (Table 2) was used. The membrane was then washed with ECL reagents and scanned using imageQuant LAS 4000 system (GE Healthcare). Images were transferred to ImageJ 1.51s for slight adjustments of contrast and brightness. For further information about the western blot reagents see Table 3.
Several controls were used in this study. Loading controls for the total amount of protein were performed using anti-Histone H3 or anti-Actin antibodies. A Δmdh2 strain, alongside Δmdh1 and Δmdh3 strains, were used to verify the specificity of the Mdh2 antibodies. An anti-mCherry antibody was used as a control for the bands detected in Mdh2-mCherry or Tef2-mCherry-Mdh3 (over expression) compared to the bands detected by the anti-Mdh2 antibodies. An anti-GFP antibody was used as a control for the bands detected in GFP-Mdh1, GFP-Mdh2 and GFP-Mdh3 with anti Mdh2 antibody peptide #3.
For first examination of the three antibodies, we grew control cells in three different carbon sources: glucose, galactose and oleic acid. Since transcription of S. cerevisiae Mdh2 is repressed by glucose8, we hypothesized that the antibodies will not detect Mdh2 in extracts of cells grown in glucose, but will detect it in the galactose and oleic acid samples. We then performed protein extraction using the NaOH approach, and examined the three GenScript anti-Mdh2 antibodies.
Indeed, all three antibodies could not detect a specific band at the correct size of Mdh2 (41 KDa) in protein extractions from cells grown in glucose (Figure 1, Glu). Although the general protein levels of the control strain grown in oleic acid were much lower than from the levels of controls grown in glucose, a prominent band at the correct size for Mdh2 was detected by all three antibodies. In galactose there was no prominent band at the correct size, although this could be due to the general low levels of protein in this condition, as can be seen by the Histone H3 loading control. Endogenous Mdh2 was also recognized in protein extractions from cells grown on other carbon sources such as EtOH (not shown). This suggests that the upregulation of Mdh2 in cells grown in oleic acid is due to the removal of the glucose repression and not because of an oleic acid-specific up-regulation.
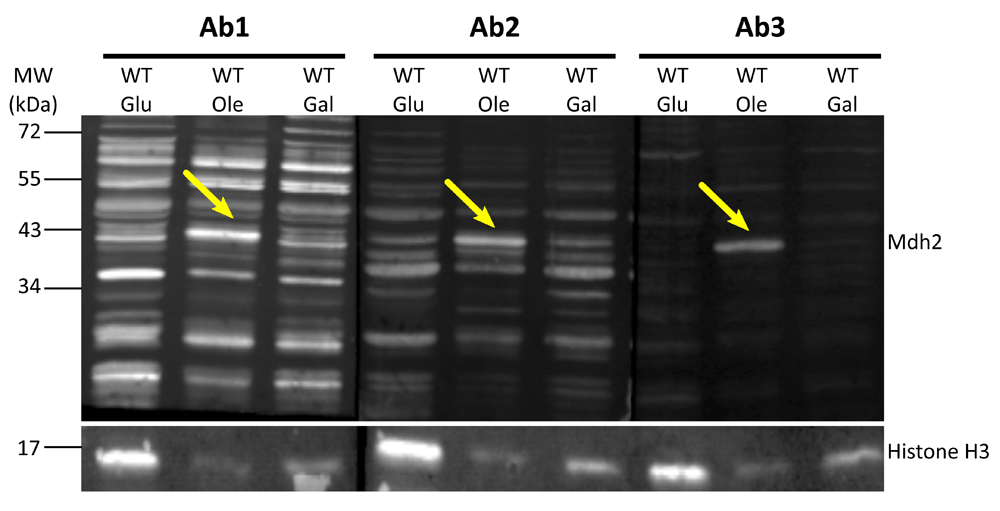
Western blot analysis was performed on protein extracts of control cells (BY4741) grown in glucose (Glu), oleic acid (Ole) or galactose (Gal) using antibodies 1–3 (Ab1, Ab2, Ab3). Native Mdh2 (41 KDa) is detected by all three anti-Mdh2 antibodies in protein extractions from cells grown in oleic acid as indicated by yellow arrows. Histone H3 was used as a loading control. In total we detected seven times a band at the size of Mdh2 when cells were grown on Oleate: One time using antibody1, three times using antibody 2 and three times using antibody 3.
After the first comparison, we focused on antibodies 2 and 3 as they displayed the lowest background signal. We verified the affinity and specificity of the antibodies using several strains, in addition to the control strain: Δmdh1, Δmdh2, Δmdh3, as well as strains that had a mCherry tag fused to either Mdh2 or Mdh3 or GFP tag fused to Mdh1, Mdh2 or Mdh3. Both antibody 2 and antibody 3 detected endogenous Mdh2 in wild type cells and in Δmdh3 cells grown in oleic acid (Figure 2 and Figure 3), but were specific to Mdh2 as they did not cross react in the Δmdh2 strain. Another way to demonstrate specificity is to tag Mdh2 with mCherry, thus shifting only this specific isoform in size. Indeed, under these conditions both antibodies 2 and 3 detected a higher protein form only (Mdh2 tagged with mCherry = ∼70 kDa), though with lower affinity. Reciprocally, neither antibody detected over expressed mCherry-Mdh3 (expressed under a Tef2 promotor), although it was highly expressed as could be verified by an anti-mCherry antibody. In addition, antibody 3 detected endogenous Mdh2 in Δmdh1 cells grown in oleic acid, as well as a bigger band size only in GFP-Mdh2 tagged cells (Mdh2 tagged with GFP = ∼68 kDa) (Figure 4). Although both antibodies fit the requirement of specific identification of the yeast Mdh2, we have decided to use antibody 3, as it has a better specificity as seen from the lower background signal (Figure 3).
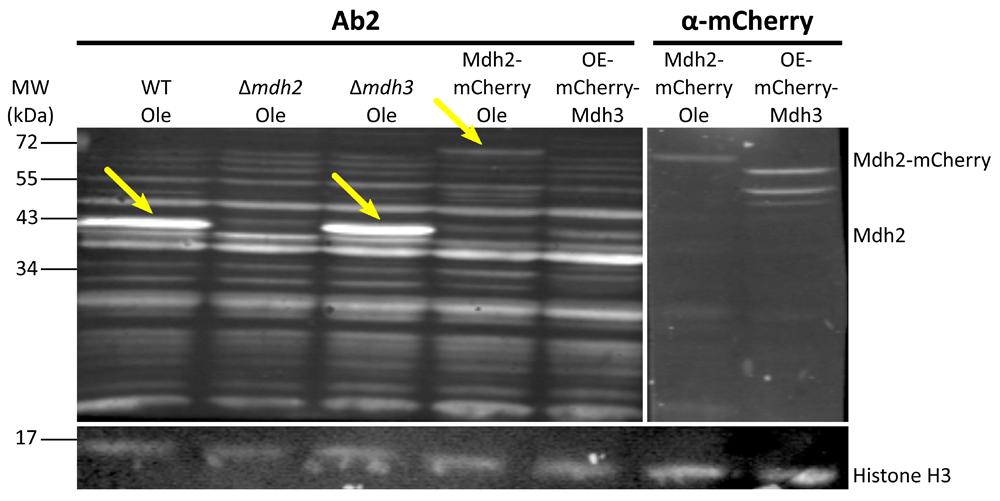
Western blot analysis was performed on protein extractions from different strains grown on oleic acid (Ole) or in glucose (only for OE-mCherry-Mdh3) using antibody 2 and anti-mCherry antibody. Antibody 2 recognizes the native Mdh2 in control cells and in Δmdh3 cells grown in oleic acid, but does not recognize it in Δmdh2 strain (second lane). The antibody also recognizes Mdh2 tagged with mCherry (∼70 kDa), but not the over-expressed and mCherry tagged Mdh3. The last one can be recognized in the control with the anti-mCherry. Histone H3 was used as a loading control. Yellow arrows indicate bands corresponding to Mdh2. (OE = over expression). We saw that antibody 2 specifically recognized Mdh2 and not Mdh3 in three experiments.
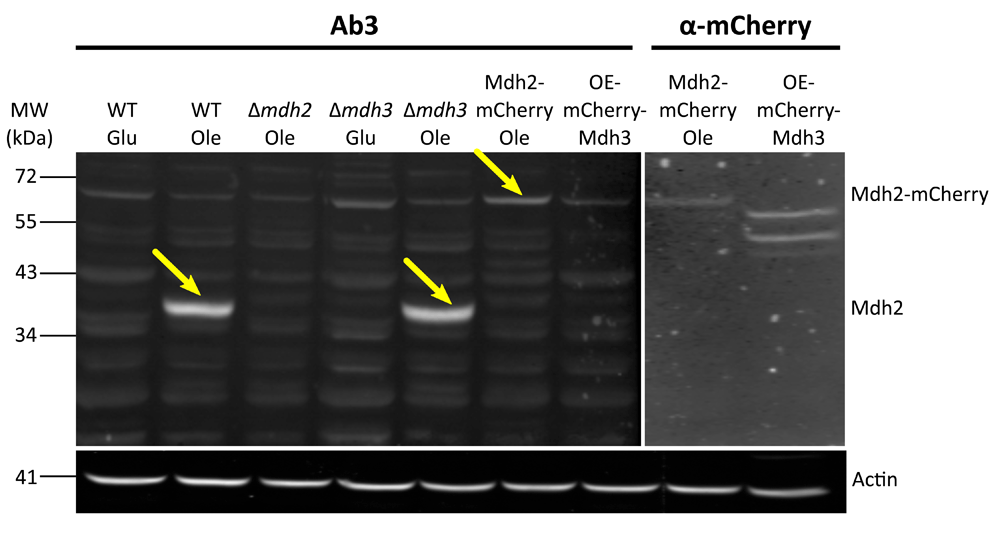
Western blot analysis was done on protein extractions from different strains grown on oleic acid (Ole) or glucose (Glu) (OE-mCherry-Mdh3 was grown in glucose) using antibody 3 and anti-mCherry. Similarly to antibody 2, antibody 3 does not recognize Mdh2 in Δmdh2 strain or in cells grown on glucose. This antibody has a good specificity to Mdh2, as seen by the low background signal. Actin was used as a loading control. Yellow arrows indicate bands corresponding to Mdh2. (OE = over expression). We saw that antibody 3 specifically recognizes Mdh2 in three experiments.
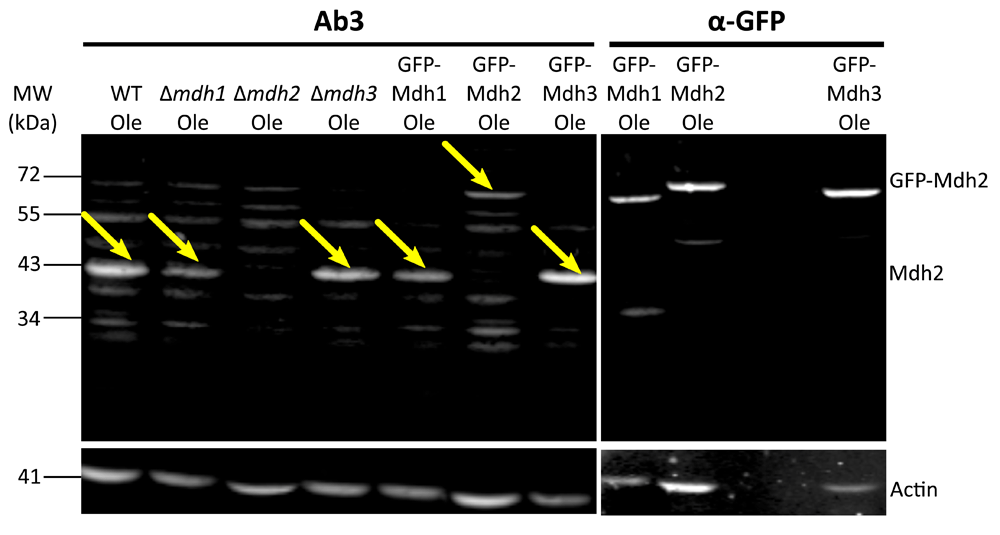
Western blot analysis was done on protein extractions from different strains grown on oleic acid (Ole) using antibody 3 and an anti-GFP antibody. As shown in Figure 3, this antibody does not recognize Mdh2 in Δmdh2 strain or in cells grown on glucose but does recognize Mdh2 in Δmdh3 cells grown on oleic acid. Here we show that antibody 3 also recognizes Mdh2 in Δmdh1 cells grown on oleic acid. Actin was used as a loading control. Yellow arrows indicate bands corresponding to Mdh2. We saw that antibody 3 specifically recognizes Mdh2 and not Mdh3 in two experiments and that antibody 3 does not recognize Mdh1 in one experiment.
Three antibodies against endogenous S. cerevisiae Mdh2 were prepared by GenScript, using specific peptides of Mdh2 as the immunogens. The antibodies were checked by western blot and were shown to have the ability to detect endogenous Mdh2, when cells are grown under conditions in which Mdh2 is expressed. Although two of the three antibodies demonstrated the specificity qualities required from such an antibody, antibody 3 had the best specificity qualities, and the lowest background signal. We thus recommend antibody 3 as the best option to detect endogenous S. cerevisiae Mdh2. This antibody can be used in western blot, as shown in this manuscript, and should be tested for other uses (e.g. ELISA, FACS, IP).
Dataset 1: raw images of all western blots included in figures presented 10.5256/f1000research.13396.d1908959
This work was supported by a European Union ERC CoG #646604 and an SFB 1190 from the DFG. MS is an incumbent of the Dr. Gilbert Omenn and Martha Darling Professorial Chair in Molecular Genetics.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank Pierce Feng from GenScript for help in antigen design and for rapid turnover time from order to delivery.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Are sufficient details of materials, methods and analysis provided to allow replication by others?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast molecular cell biology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Are sufficient details of materials, methods and analysis provided to allow replication by others?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Organelle biogenesis, glyoxylate cycle, yeast
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 30 Jul 18 |
||
|
Version 1 31 Jan 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)