Keywords
Passive smoking, COPD, Dose-response, Meta-Analysis, Review, Pulmonary Disease
Passive smoking, COPD, Dose-response, Meta-Analysis, Review, Pulmonary Disease
The text has been amended to make clearer that the evidence strongly suggests - but does not definitively prove - that ETS increases COPD risk. Amendments have been made:
(i) in the conclusions section of the abstract where we now say "strongly suggests" rather than "suggests",
(ii) in the section "Comparison with other recently published reviews" in the Discussion, where the final paragraph now starts "Generally these reviews point to an association between ETS exposure and risk of COPD without concluding that a causal relationship has clearly been established. The present review confirms the association and provides evidence that is strongly suggestive of a true effect". It then ends with a sentence "While this suggestion is not inconsistent with the view of the Global Burden of Disease Study 2017 (Reference added) that second-hand smoke is a risk factor for COPD, limitations of the evidence, discussed above, preclude a more definitive conclusion" which refers to a recent study mentioned by one of the reviewers.
(iii) at the start of the Conclusions section after the Discussion which now begins "Taken in conjunction with the strong association of smoking with COPD, the significant relationship seen for the main index of ETS exposure, and the evidence of a dose-response relationship is high;y suggestive that ETS also increases risk of COPD. However, the absence of....."
The two referees are now thanked in the Acknowledgement Section.
See the authors' detailed response to the review by Yousser Mohammad
See the authors' detailed response to the review by Sara Maio
This systematic review aims to present an up-to-date meta-analysis of available epidemiological evidence relating exposure to environmental tobacco smoke (ETS) from cigarettes to risk of chronic obstructive pulmonary disease (COPD) in lifelong non-smokers (“never smokers”). As described below, this review considers data from 28 longitudinal, case-control or cross-sectional studies1–28.
It is long established that active smoking causes COPD, the U.S. Surgeon General concluding in 196429 that “cigarette smoking is the most important of the causes of chronic bronchitis in the United States, and increases the risk of dying from chronic bronchitis”. This opinion was echoed in their 2004 report30, which felt the evidence “sufficient to infer a causal relationship between active smoking and chronic obstructive pulmonary disease morbidity and mortality”, a view confirmed by a recent systematic review31.
Sidestream smoke (released between puffs from the burning cone) contains similar chemicals to mainstream smoke (drawn and inhaled by smokers), but with different relative and absolute quantities of many individual constituents32. However, sidestream smoke, after mixing with aged exhaled mainstream smoke, is diluted massively by room air before non-smokers inhale it. Smoke constituent levels in tissues of non-smokers are very much lower than in smokers, studies using cotinine typically indicating a relative exposure factor between 0.06% and 0.4%33–35, with studies using particulate matter indicating a lower factor of 0.005% to 0.02%36–44. Though an effect of ETS on COPD risk is plausible, it is difficult to establish this with certainty, as a threshold is a logical possibility. The same difficulty of establishing effects of ETS exposure on other diseases caused by smoking is also present, notably for lung cancer31,45.
In 2006, a review by the U.S. Surgeon General of the association of COPD with ETS exposure46 concluded that “the evidence is suggestive but not sufficient to infer a causal relationship between second-hand smoke exposure and risk for COPD”, the need for additional research also being highlighted. Although that review cited only nine of the 28 studies considered here1,3,5–10,13, and although various new studies have appeared since then, no other fully comprehensive review of this subject appears to have been undertaken.
This review, which is essentially an update of the 2006 review46 is an attempt to assess the epidemiological evidence currently available, restricting attention to studies of COPD in which its relationship to one or more ETS exposure indices has been studied in never smokers. This restriction to never smokers is necessary as there is a very strong association of COPD with smoking46, and it is difficult to reliably detect any ETS effect where a history of smoking is present. This is because the extent of a smoker’s overall exposure to smoke constituents is determined largely by his own smoking habits and hardly at all by his much smaller ETS exposure, and also because smoking and ETS exposure are correlated (e.g. since smokers tend to marry smokers). Any errors in assessing smoking history are therefore likely to cause a residual confounding effect much larger than any plausible ETS effect47.
As the 2006 US Surgeon General’s Report46 notes “COPD is a non-specific term, defined differently by clinicians, pathologists, and epidemiologists, each using different criteria based on symptoms, physiologic impairment, and pathologic abnormalities”. That report goes on to state that “the hallmark of COPD is the slowing of expiratory airflow measured by spirometric testing, with a persistently low FEV1 [forced expiratory volume in one second] and a low ratio of FEV1 to FVC [forced vital capacity] despite treatment”. International guidelines48 define COPD as post-bronchodilator FEV1/FVC <0.70, with severity classified by subdividing FEV1 as a percentage of predicted into four groups (≥80, <80, <50 and <30%). The term COPD was little used until the 1980s, and diagnoses commonly used earlier (e.g. chronic bronchitis and emphysema) do not correspond exactly to what is now termed COPD. The studies we selected for review used disease definitions close enough to COPD as now defined to reasonably allow overall assessment. Some studies present additional results using criteria corresponding to severer forms of the disease. While these data are presented here, they are not included in our detailed meta-analyses.
This systematic review was conducted according to PRISMA guidelines49.
Attention is restricted to epidemiological longitudinal, case-control or cross-sectional studies which provide risk estimates for never (or virtually never) smokers for any of the following indices of ETS exposure: spouse, partner, cohabitant, at home, at work, in adulthood, in childhood.
The term COPD is relatively recent, so we also included studies with outcomes described otherwise. Following the strategy used in our review of smoking and COPD31, outcomes “could be based on International Classification of Diseases (ICD) codes, on lung function criteria, on a combination of lung function criteria and symptoms, or on combinations of diagnosed conditions….where diagnoses were extracted from medical records or reported in questionnaires”. Acceptable combinations of diagnosed conditions had to include both chronic bronchitis and emphysema, but could also additionally include asthma, acute and unqualified bronchitis or bronchiectasis. However, studies were rejected where results were only available for emphysema, for chronic bronchitis, for respiratory symptoms such as cough or phlegm, or for lung function criteria not equating to COPD. Over-broad definitions such as respiratory disease were also not accepted. Acceptable lung function criteria included those of the Global Initiative for Chronic Obstructive Lung Disease (GOLD), the European Respiratory Society, and the British and American Thoracic Societies,
Studies which provide near equivalent definitions of “never smokers” are also accepted; thus never smokers can include occasional smokers or smokers with a minimal lifetime duration of smoking or number smoked. Risk estimates may be based on relative risks (RRs), hazard ratios (HRs), or odds ratios (ORs), and must either be provided directly or be capable of being estimated from the data provided.
A PubMed search identified papers published up to June 2016 using the term “COPD AND (ENVIRONMENTAL TOBACCO SMOKE OR PASSIVE SMOKING OR SECONDHAND SMOKE EXPOSURE OR INVOLUNTARY SMOKING)”, with restriction to humans. After rejecting papers that were clearly irrelevant based on the abstract, copies of the others were obtained for inspection. Other potentially relevant papers were obtained from reference lists in the 2006 Surgeon General report46, an earlier review we conducted47 and relevant review papers identified in the search. The complete list of potentially relevant papers were then looked at in detail to determine those which described studies satisfying the selection criteria, the rejected papers also including those where an alternative paper provided results from the same study that were more useful (e.g. based on a longer follow-up, a larger number of cases, or using a disease definition closer to COPD as currently defined).
Details were extracted from relevant publication on the following: study author; year of publication; study location; study design; sexes included; disease definition; number of cases; potential confounding variables considered; and never smoker definition. An effect estimate together with its associated 95% confidence interval (CI) was obtained, where available, for ETS exposure at home, at work, in adulthood, childhood, and from these sources combined. Choice between multiple definitions of COPD followed the rules of Forey et al.31, except that here we also obtained additional estimates, if available, for severer COPD. We preferred effect estimates where the denominator was with no (or minimal) exposure to the ETS type considered rather than with no exposure to any ETS. Effect estimates and 95% CIs extracted were sex-specific, if possible, and for longitudinal studies were for the longest follow-up available. Estimates adjusted for covariates, where available, were generally preferred to unadjusted estimates, except that results adjusted for symptoms or precursors of COPD were not considered. Where a study provided multiple adjusted estimates, we used that adjusted for most covariates. Dose-response data were also extracted, where available.
For a study reporting effect estimates and CIs only by exposure level, that for the overall unexposed/exposed comparison was estimated using the Morris and Gardner method50 for unadjusted data or the Hamling et al. method51 for adjusted data. These methods also allowed estimation of the significance of dose-related trends, if not given in the source publication.
As the great majority of effect estimates were ORs derived from case-control or cross-sectional studies, and as the RRs or HRs from longitudinal studies were all based on low incidences, where the OR would be virtually the same, all estimates were treated as if they were ORs. In the rest of this paper, we use OR rather than referring to specific types of effect estimate.
A pre-planned set of fixed-effect and random-effects meta-analyses were carried out using standard methods52. Heterogeneity was quantified by H, the ratio of the heterogeneity chi squared to its degrees of freedom. The I-squared statistic53 is related to H by the formula I2 = 100 (H-1)/H. Publication bias tests were also conducted using the Egger method54.
Our main analyses included OR estimates for the exposure most closely equivalent to “spouse ever smoked” where results were provided or could be estimated. This selection was based on the source of exposure (spouse highest preference, then partner, cohabitant, home or work). Spousal smoking is traditionally used for studying possible ETS effects, it being clearly demonstrated that women married to a smoker have much higher cotinine levels than women married to a non-smoker55. Apart from the meta-analyses using all available estimates, meta-analyses also investigated variation in the OR according to a list of pre-defined factors, and using the following subsets: continent (North America, Asia, Europe, multicountry); publication period (1976–1990, 1991–2005, 2006–2016); study type (longitudinal, case-control, cross-sectional); sex (males, females, combined); diagnosis (mortality or hospitalisation, GOLD stage 1+, other); method of taking asthma into account (included as part of the COPD definition, adjusted for, asthmatic participants excluded, ignored); number of cases estimate based on (<50, 50–149, 150+ cases); extent of confounder adjustment (unadjusted for age, adjusted for age and at most four other variables, adjusted for age and five or more variables); never smoker definition (never smoked any product, never smoked but product unstated, other – including never cigarette smoker, occasional smoker or very short-term smoker); and definition of exposure index (spouse specifically, other exposure at home, other).
Meta-analyses were also carried out for the main index using the estimates for severer COPD, and also for other indices of exposure with sufficient data (workplace, overall adult – including at least home and work, childhood). Here, data were too limited to study variation in the OR by the subsets described above.
Results of the overall meta-analyses are displayed as forest plots. In each plot, individual estimates are listed in increasing order of the OR. For the main index, estimates are grouped by region. Random-effects estimates are also shown. The estimates are not only shown numerically, but in graphical form on a logarithmic scale, where the OR is shown as a square, the area of which is proportional to its inverse-variance weight. Arrows warn when the CI goes outside the range of the plot.
We did not attempt to derive any overall score based on study quality and risk of bias for each individual study, as the relative importance of different sources of bias or poor study quality is difficult or impossible to assess accurately. Instead, we attempted to gain insight into this in two ways. First, as mentioned in the previous section, we carried out meta-analyses showing how the OR varied by some relevant aspects linked to study quality and bias, such as study size, study type, source of diagnosis, method of taking asthma into account, and extent of confounder adjustment. Second, we considered factors affecting quality and bias in the discussion section, including some factors that affected all or virtually all of the studies.
The PubMed search produced 509 hits. As summarized in Figure 1, Seventy-five were considered of potential relevance based on the abstracts, 15 of which proved to meet the inclusion criteria on examination of the papers themselves. Further examination of reference lists in reviews46,47,56–63 and in papers obtained identified a further 40 papers of potential relevance, 13 of which met the inclusion criteria. Of the 87 papers examined but not accepted, the most common reasons for rejection were no results for never smokers (38 papers), not COPD as defined (26), no control group or no results for unexposed participants (11) and better results for the same cohort given in another paper (9), some studies being rejected for more than one reason. Supplementary File 1 gives details of the studies rejected and fuller reasons for rejection.
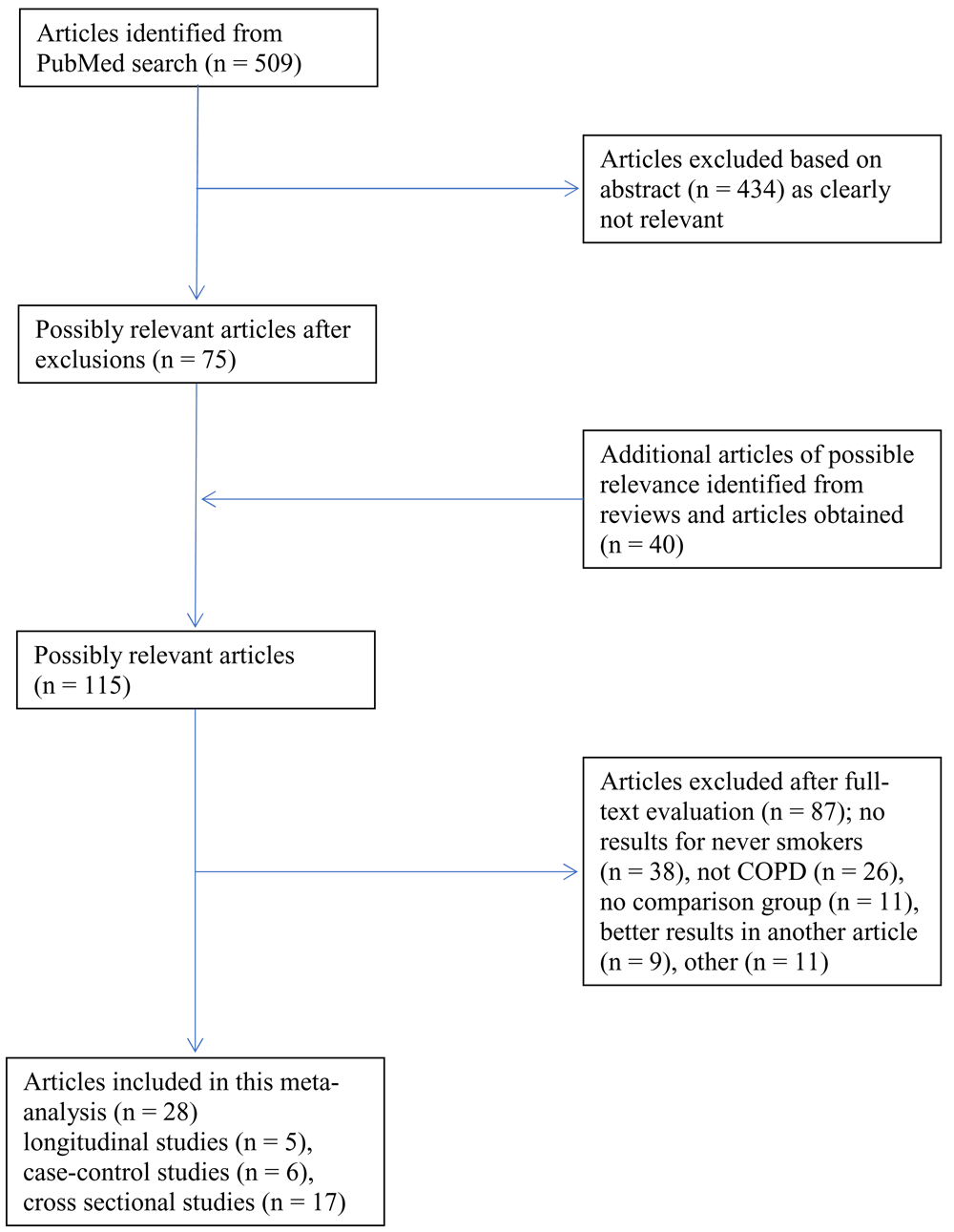
The flow-chart shows the number of articles identified from the PubMed search and from reference lists of reviews and articles obtained, as well as showing those excluded, with reasons for exclusion. Note that some articles were excluded for multiple reasons.
Table 1 gives details of the 28 epidemiological studies that met the inclusion criteria, including author, reference(s), publication year, location, design, sexes included, disease definition, account taken of asthma, and numbers of cases in never smokers. The studies are listed in chronological order of publication and are given consecutive identifying study numbers.
| Study No. | Authora | Yearb | Location | Typec | Sexes included | Age ranged | Definition of COPD usede | Accounting for asthma | No. of casesf |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lebowitz1 | 1976 | USA | CS | M,F | 15+ | Asthma, bronchial trouble or emphysema (physician diagnosis, questionnaire report) | Included | 246 |
| 2 | Comstock2 | 1981 | USA | CS | Mg | 20+ | FEV1/FVC <0.70 (spirometry testh) | Ignored | 30 |
| 3 | Hirayama3 | 1984 | Japan | L15 | F | 40+ | Emphysema or chronic bronchitis (mortality) | Ignored | 130 |
| 4 | Krzyzanowski4 | 1986 | Poland | L13 | M,F | 19–70 | FEV1 <65% predicted (spirometry testh) | Ignored | 37 |
| 5 | Lee5 | 1986 | England | CC | M,F | 35–74 | Chronic bronchitisi (hospitalisation) | Ignored | 26 |
| 6 | Kalandidi6 | 1987 | Greece | CC | F | 40–79 | Chronic obstructive lung disease (hospitalisation) | Excludedj | 103 |
| 7 | Sandler7 | 1989 | USA | L12 | M,F | 16+ | Emphysema or bronchitis (mortality) | Ignored | 19 |
| 8 | Dayal8 | 1994 | USA | CSk | M,F | Adults | Chronic bronchitis, emphysema or asthma (diagnosis, questionnaire report) | Included | 219 |
| 9 | Forastiere9 | 2000 | Italy | CSl | F | 25–74 | COPD (physician diagnosis, questionnaire report) | Ignored | 50 |
| 10 | Enstrom10 | 2003 | USA | L39 | M,F | 31+ | COPD (mortality) | Included | 264 |
| 11 | De Marco11 | 2004 | 16 countries | CS | M,F | 20–44 | COPD (GOLD stage 1+)h | Ignored | 156 |
| 12 | Celli12 | 2005 | USA | CS | M,F | 30–80 | FEV1/FVC <0.70 (spirometry testh) | Ignored | 414m |
| 13 | McGhee13 | 2005 | Hong Kong | CC | M,F | 60+ | COPD (including pulmonary heart disease, mortality) | Ignored | 138 |
| 14 | Sezer14 | 2006 | Turkey | CC | F | 38+ | COPD (specialist clinic diagnosis) | Ignored | 74 |
| 15 | Xu15 | 2007 | China | CC | M,F | 35+ | COPD (hospital diagnosis of emphysema or chronic bronchitis, questionnaire report) | Ignored | 1097 |
| 16 | Yin16 | 2007 | China | CS | M,F | 51+ | COPD (GOLD stage 1+ but without bronchodilator) | Excluded | 429 |
| 17 | Zhou17 | 2009 | China | CS | M,F | 40+ | COPD (GOLD stage 1+)n,o | Ignored | 644 |
| 18 | Wu18 | 2010 | Taiwan | CC | F | 40+ | COPD (GOLD stage 1+) | Excluded | 168 |
| 19 | Jordan19 | 2011 | England | CS | M,F | 40+ | COPD (GOLD stage 1+ but without bronchodilator)p | Excluded | 779m |
| 20 | Lamprecht20 | 2011 | 14 countries | CS | M,F | 40+ | COPD (GOLD stage 1+)n,q | Adjusted | 523 |
| 21 | Chen21 | 2012 | China | CS | M,F | 60+ | COPD (physician diagnosis, questionnaire report) | Ignored | 149 |
| 22 | He22 | 2012 | China | L17 | M,F | 51–87 | COPD (mortality or GOLD stage 1+)r | Ignored | 36 |
| 23 | Waked23 | 2012 | Lebanon | CS | M,F | 40+ | COPD (GOLD stage 1+) | Ignored | 25 |
| 24 | Moreira24 | 2013 | Brazil | CS | F | 40+ | COPD (GOLD stage 1+) | Excluded | 43 |
| 25 | Eze25 | 2014 | Switzerland | CS | M,F | 18–65 | COPD (GOLD stage 1+)h | Ignored | 444 |
| 26 | Hagstad26 | 2014 | Sweden | CS | M,F | 20–77 | COPD (GOLD stage 1+) | Ignored | 41m |
| 27 | Kim27 | 2014 | Korea | CS | M,F | 40+ | COPD (GOLD stage 1+ but without bronchodilator) | Adjusted | 323 |
| 28 | Tan28 | 2015 | Canada | CS | M,F | 40+ | COPD (LLN, FEV1/FVC <5th centile)s | Adjusted | 161 |
a First author and reference of principal publication
b Year of publication
c Study types are CC = case-control, CS = cross-sectional, L = longitudinal. For longitudinal studies, number of years follow-up is shown
d Age at baseline for longitudinal studies
e Definition of principal COPD outcome is shown. Definition of severer COPD used is shown in footnotes, along with alternative definitions for which results are available
f Number of cases in lifelong non-smokers
g Study also included females, but none had COPD
h No mention of use of bronchodilator prior to spirometry
i Named as chronic bronchitis, but defined by authors as ICD 9th revision 491, 492, 496 so equates to COPD
j Ignored for controls
k Analysed as a nested CC study
l Never smoking women had been identified by earlier studies in the same areas
m Approximate estimate
n Severer outcome definition based on GOLD Stage 2+
o Alternative results are also available for GOLD stage 0+
p Severer outcome definition based on NICE criteria (FEV1/FVC <0.7 and FEV1 <80% predicted) described as equivalent to GOLD stage 2+ (no bronchodilator, omitting participants with diagnosis of asthma). Alternative results are also available based on the LLN criteria, for “clinically significant COPD” based on the LLN, GOLD and NICE criteria, and including participants with diagnosis of asthma
q Alternative results are also available using the LLN criteria
r Based on death certificate, supplemented by medical records for lung function if their death was not from COPD
s Severer outcome definition is based on LLN criteria plus FEV1<80% predicted
The included studies are mainly of representative populations, except that studies 18 and 26 have a large proportion with respiratory symptoms. Of the 28 studies, one was published in the 1970s, six in the 1980s, one in the 1990s, nine between 2000 and 2009 and 11 more recently.
Nine studies were conducted in Europe or the Middle East, subsequently referred to as “Europe” (two in England, and one each in Greece, Italy, Lebanon, Poland, Sweden, Switzerland and Turkey), while nine took place in Asia (five in China, and one each in Hong Kong, Japan, Korea and Taiwan), seven in North America (six in the USA and one in Canada) and one in South America (Brazil). Two studies presented combined results, one from 16 countries, the other from 14.
Five studies were longitudinal in design, with the length of follow-up varying from 12 to 39 years, one was a cross-sectional study analysed as a nested case-control study, 16 other studies were cross-sectional, with the remaining six of case-control design.
Most studies were of both sexes, though six studies considered only females.
Two studies considered those with a minimum age of 60, with a further 16 having a minimum age between 35 and 51. Other studies had a lower minimum age.
Definitions of outcome used varied by study. Seven studies required the case to have died or been hospitalised for COPD, while a further 12, mainly relatively recent cross-sectional studies, used COPD as defined by the GOLD stage 1+ criteria. The remaining nine studies used other definitions, as detailed in Table 1. Five studies (17, 19, 20, 26, 28) also provided results for severer COPD (generally equivalent to GOLD 2+, see footnotes to Table 1).
Twenty-one studies ignored asthma in their outcome definition and analysis, with the remaining 12 studies equally divided into those that included asthma in their outcome definition, excluded asthmatics, or adjusted for asthma status in analysis.
Most studies were small, with ten studies considering less than 100 cases and only one study (15) more than 1000 cases.
Table 2 gives the adjustment variables used and the definitions of never smokers used in the studies.
| Study No. | Author | Variables adjusted for | Definition of never smokersa |
|---|---|---|---|
| 1 | Lebowitz | None | Never NOS |
| 2 | Comstock | Age, education, number of bathrooms, persons/room, children in household, air conditioning, cooking fuel | Never cigarettes |
| 3 | Hirayama | Age of husband | Never cigarettes |
| 4 | Krzyzanowski | Age | Never NOS |
| 5 | Lee | Age, marital status (Table 3) Age (Table 5, Table 6 and Table 7) | Never NOS |
| 6 | Kalandidi | Age, occupation | Never NOS |
| 7 | Sandler | Age, housing quality, schooling, marital status | Never any product |
| 8 | Dayal | Age, sex, neighbourhood, heating, cooking | Never NOS |
| 9 | Forastiere | Age, center, age x center, education | Never cigarettes |
| 10 | Enstrom | Ageb | Never any productc |
| 11 | De Marco | Sex, childhood respiratory infections, occupational exposure, socioeconomic status | Never smoked 20 packs of cigarettes or 360 g of tobacco in a lifetime, or at least 1 cigarette/day or 1 cigar/week for a year |
| 12 | Celli | Age, sex, race/ethnicity, BMI, education, poverty, urban residence, high risk industry, high risk occupation, biomass, allergy | Never smoked 100 cigarettes in lifetime |
| 13 | McGhee | Age, education (Table 3) Age, sex, education (Table 4) | Never NOS |
| 14 | Sezer | Wood ash, biomassd | Never NOS |
| 15 | Xu | Education, occupation, family income, cooking fuels, heating in winter, ventilating fans, occupational physical activity | Never NOS |
| 16 | Yin | Age, sex, education, occupational dust exposure, indoor air pollution | Never NOS |
| 17 | Zhou | Age, sex, education, BMI, family history of respiratory disease, biomass, heating fuel, ventilation in kitchen, childhood chronic cough, occupational exposures | Never NOS |
| 18 | Wu | Age, height, education level, cooking status, burning incense, tea consumption | Never smoked, on average, more than 1 cigarette/day for a year |
| 19 | Jordan | Age, sex, year of study | Never smoked at least 1 cigarette/day |
| 20 | Lamprecht | None (COPD in Table 3) Age, education, occupational exposure, biomass fuel use, childhood hospitalisation, comorbidity, BMI (severer COPD in Table 3) | Never smoked more than 20 packs in lifetime or more than 1 cigarette/day for a year |
| 21 | Chen | None | Never cigarettes |
| 22 | He | Age, sex, marital status, occupation, education, alcohol, diastolic blood pressure, triglyceride and total cholesterol levels, BMI | Never smoked 100 cigarettes in lifetime |
| 23 | Waked | Age, sex, area of residence | Never NOS |
| 24 | Moreira | None | Never NOS |
| 25 | Eze | None | Never NOS |
| 26 | Hagstad | Age, sex, asthma, family history of obstructive airway disease, socioeconomic group (COPD in Table 3) None (severer COPD in Table 3 and Ever home/work and Childhood in Table 6) | Never smoked more than 1 cigarette/day for a year |
| 27 | Kim | Age, sex, previous diagnosis of asthma or tuberculosis, family income, education status | Never cigarettes |
| 28 | Tan | Age, education, childhood respiratory illness, heart disease, hypertension or diabetes, asthma, BMI, exposure to organic dust, inorganic dust, gases and vapours, biomass cooking and heating for ≥ 10 years, TB | Never smoked more than 1 cigarette/day for a year |
a Never any product = never smoked cigarettes, pipes or cigars; Never NOS = never smoked, product unspecified;
b Results adjusted for more variables not used as adjustment included health status
c Questions on pipe and cigar smoking were asked at baseline, but not at the follow-up interviews
d The cases and controls were matched on age
Five studies (1, 20, 21, 24, 25) made no adjustment for any potential confounding variables, while some others made little or no adjustment for such variables as occupation, education, diet and family history of disease, which may differ between smoking and non-smoking households64. Failure to adjust for household size, where the index of exposure is based on presence of a smoker in the household, was also common. Where adjustment was carried out, all but four studies considered age, although study 3 adjusted for the husband’s age rather than the subject’s.
Fifteen studies were of never smokers, though only three of these made it clear they were never smokers of cigarettes, pipes or cigars. Five studies were of never cigarette smokers (i.e. they may have included some pipe or cigar only smokers), the remaining eight allowing a minimal smoking history, such as smoking less than 1 cigarette a day or less than 100 cigarettes in life.
The main meta-analyses use an exposure index that relates as closely as possible to ever smoking by the spouse. Table 3 shows the definitions of ETS exposure used for the main index. This was based on smoking by the spouse for five studies, and on smoking by cohabitants for a further 13 (although study 13 only included participants who had lived with a smoker 10 years previously, and study 20 only considered ETS exposure in the home in the two weeks prior to the study). For the remaining studies, the index was based on exposure in the home and at work (studies 4, 12, 17, 18 and 27) or on a combination of exposure from any source (studies 11, 15, 19, 21 and 25).
| Study No. | Author | Typeb | Sex | Definition of exposurec | Number of cases | Odds ratio (95% CI)d | |
|---|---|---|---|---|---|---|---|
| Unexposed | Exposed | ||||||
| COPD: | |||||||
| 1 | Lebowitz | CS | M+F | Lives with ever smokere | 129 | 117 | 1.09 (0.83-1.44)f |
| 2 | Comstock | CS | M | Lives with a smoker | 23 | 7 | 1.19 (0.50-2.86)f |
| 3 | Hirayama | L15 | F | Husband ever smoked | 28 | 102 | 1.38 (0.86-2.21)f |
| 4 | Krzyzanowski | L13 | F | Exposure at home or workplace | 26 | 6 | 0.36 (0.15-0.86)f |
| M | 3 | 2 | 1.39 (0.26-7.40)f | ||||
| 5 | Lee | CC | F | Spouse smoked in marriage | 4 | 13 | 1.22 (0.38-3.94)f |
| M | 8 | 1 | 0.34 (0.06-2.03)f | ||||
| 6 | Kalandidi | CC | F | Husband ever smokede | 13 | 90 | 1.38 (0.69-2.76)f |
| 7 | Sandler | L12 | F | Lived with a smoker | 2 | 11 | 5.65 (1.19-26.8) |
| M | 4 | 2 | 0.93 (0.16-5.32) | ||||
| 8 | Dayal | CS | M+F | Lives with a smoker | 74g | 145g | 1.40 (0.98-1.99)f |
| 9 | Forastiere | CS | F | Ever married to a cigarette smoker | 11 | 39 | 1.75 (0.88-3.47) |
| 10 | Enstrom | L39 | F | Spouse ever smokede | 45 | 128 | 1.13 (0.80-1.58) |
| M | 69 | 22 | 1.27 (0.78-2.08) | ||||
| 11 | De Marco | CS | M+F | 4+ hours per day exposure on most days/nights in previous 12 months | 129 | 27 | 1.14 (0.74-1.77)f |
| 12 | Celli | CS | M+F | Lives with a smoker who smokes in the home, or exposed at work at least 1 hour per day | 327g | 86g | 0.88 (0.57-1.36) |
| 13 | McGhee | CC | F | Lived with a smoker 10 yrs ago | 15 | 27 | 2.90 (1.34-6.29) |
| M | 69 | 27 | 1.67 (0.95-2.94) | ||||
| 14 | Sezer | CC | F | Lived with a smoker for at least 10 yrs | 13 | 61 | 2.57 (1.04-6.36)f |
| 15 | Xu | CC | M+F | Spent 15+ minutes, 3+ times per week in room with smoker at any time in life | Total 1097 | 0.95 (0.79-1.16) | |
| 16 | Yin | CS | M+F | Lived with a smoker | 195 | 234 | 0.95 (0.77-1.18)f |
| 17 | Zhou | CS | M+F | Exposure at home or workplace | 119g | 525g | 1.34 (1.08-1.65)f |
| 18 | Wu | CC | F | Exposure at home (including childhood) or workplace | 41 | 127 | 2.20 (1.39-3.49)f |
| 19 | Jordan | CS | M+F | 1+ hours of exposure per week | Total 779g | 1.11 (0.95-1.30)f | |
| 20 | Lamprecht | CS | M+F | Exposure at home in previous 2 weeks | 423 | 100 | 0.89 (0.70-1.20)f |
| 21 | Chen | CS | M+F | Exposure at home, workplace or other places | 85 | 64 | 1.82 (1.30-2.54)f |
| 22 | He | L17 | M+F | Exposure at home for 15+ minutes per day, 1+ days per week for 2+ yearsh | 10 | 4 | 1.67 (0.49-5.78) |
| 23 | Waked | CS | M+F | Lives with a smoker | Total 25 | 1.23 (0.55-2.74)f | |
| 24 | Moreira | CS | F | Lives with a smoker | Total 43 | No significant difference in number of years exposure, or proportion exposed to straw cigarettesi | |
| 25 | Eze | CS | M+F | Exposure at home, workplace or other places in previous year | 293 | 151 | 1.00 (0.81-1.24)f |
| 26 | Hagstad | CS | M+F | Ever lived with a smokerj | 25 | 64 | 1.38 (0.84-2.27) |
| 27 | Kim | CS | M+F | Exposure at home or workplace | 248 | 75 | 0.85 (0.60-1.21)f |
| 28 | Tan | CS | F | Lived with a smoker in previous 2 weeks | M&F 94 | M&F 12 | 2.20 (1.03-4.71) |
| M | 1.01 (0.27-3.76) | ||||||
| Severer COPDk: | |||||||
| 17 | Zhou | CS | M+F | Exposure at home or workplacej | 89 | 379 | 1.27 (1.00-1.63)f |
| 19 | Jordan | CS | M+F | 1+ hours of exposure per week | Total 334g | 1.13 (0.84-1.51)f | |
| 20 | Lamprecht | CS | F | Exposure at home in previous 2 weeks | Total 159 | 1.53 (0.98-2.41) | |
| M | Total 67 | 0.97 (0.40-2.40) | |||||
| 26 | Hagstad | CS | M+F | Ever lived with a smokerj | 11 | 35 | 2.46 (1.24-4.88)f |
| 28 | Tan | CS | F | Lives with a smoker | M&F 94 | M&F 4 | 1.65 (0.46-5.88) |
| M | 0.69 (0.08-6.31) | ||||||
a Or nearest equivalent to spouse or household member (see text and Table)
b Study types are CC = case-control, CS = cross-sectional, L = longitudinal. For longitudinal studies, number of years follow-up is shown
c Comparison is with those not exposed as defined, except where indicated otherwise
d RRs from longitudinal studies are taken as being equivalent to ORs
e Separate results also available for current smoker and exsmoker
f OR and/or CI estimated from data provided
g Approximate estimates
h Compares exposed at home only to unexposed. Excludes those exposed at work
i A straw cigarette is a handful of tobacco, wrapped in a corn husk; study not included in meta-analysis
j Compared to subjects not exposed to any source of ETS; results also available for current or former exposure
k Results not included in the meta-analysis in Figure 2
Although most studies presented results comparing participants who were exposed or unexposed to ETS, some required a minimum level before a subject could be classified as exposed. In study 19, exposure had to be for at least one hour per week, while study 12 specified living with a smoker who smoked in the home or exposure at work for at least one hour per day. In studies 20 and 28, exposure had to have been in the previous two weeks, while participants in study 25 had to have had regular exposure in the previous year. In study 22 exposure had to be for 15+ minutes per day at least once per week for two or more years, while in study 15 the minimum requirement was 15 minutes or more, three or more times per week. In study 11, participants were only considered to have been exposed if they reported four or more hours of exposure on most days or nights in the previous year. Finally, study 14 required 10 years of exposure.
Table 3, supported by Figure 2, also presents the ORs for the main exposure index, while Table 4 presents the results of meta-analyses, and Table 5 the dose-response data.
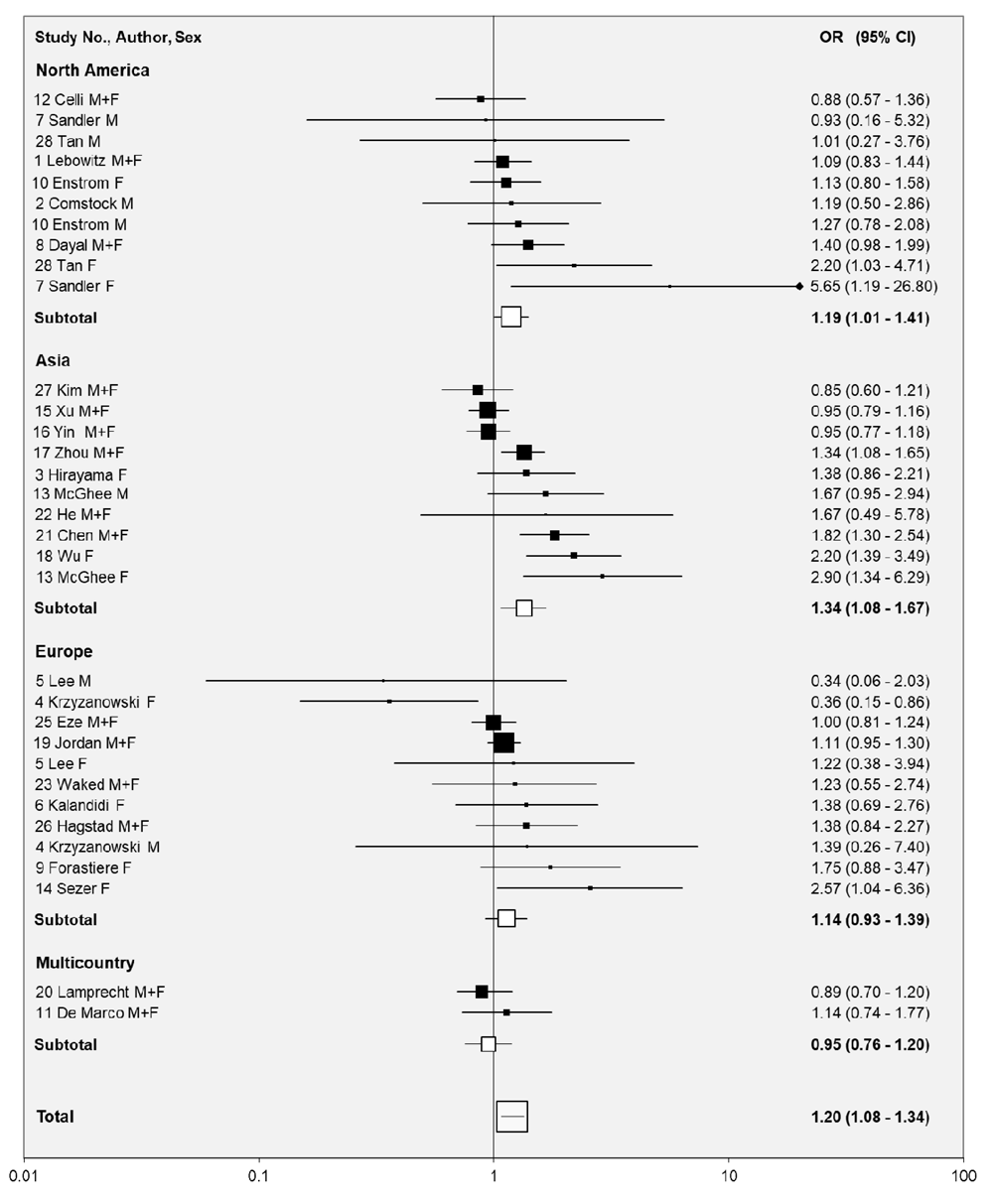
Individual study estimates of the OR and its 95%CI are shown separately by region, sorted in increasing order of OR. These are shown as numbers, and also graphically on a logarithmic scale. Random-effects estimates of ORs and 95%CIs are also shown for each region combined and overall. Studies are identified by the study number shown in Table 1. In the graphical representation, ORs are indicated by a square, with the area of the square proportional to the weight.
| Fixed-effect | Random- effects | Publication bias | Heterogeneityc | |||
|---|---|---|---|---|---|---|
| Subgroup | Nd | Odds ratio (95% CI) | Odds ratio (95% CI) | pe | Chisquared per DFf | pg |
| All COPD | 33 | 1.14 (1.07-1.21) | 1.20 (1.08-1.34) | <0.1 | 1.99 | <0.001 |
| By continent | ||||||
| North America | 10 | 1.19 (1.02-1.38) | 1.19 (1.01-1.41) | NS | 1.08 | NS |
| Asia | 10 | 1.18 (1.07-1.30) | 1.34 (1.08-1.67) | <0.1 | 3.83 | <0.001 |
| Europeh | 11 | 1.10 (0.98-1.24) | 1.14 (0.93-1.39) | NS | 1.53 | NS |
| Multicountry | 2 | 0.95 (0.76-1.20) | 0.95 (0.76-1.20) | - | 0.90 | NS |
| Between continents | 1.13 | NS | ||||
| By publication period | ||||||
| 1976–1990 | 10 | 1.12 (0.91-1.37) | 1.11 (0.81-1.52) | NS | 1.52 | NS |
| 1991–2005 | 8 | 1.28 (1.09-1.50) | 1.30 (1.07-1.59) | <0.1 | 1.40 | NS |
| 2006–2015 | 15 | 1.11 (1.03-1.20) | 1.19 (1.03-1.37) | <0.1 | 2.70 | <0.001 |
| Between periods | 1.18 | NS | ||||
| By study type | ||||||
| Longitudinal | 8 | 1.18 (0.94-1.47) | 1.18 (0.83-1.67) | NS | 1.71 | NS |
| Case-control | 8 | 1.20 (1.02-1.40) | 1.55 (1.04-2.32) | NS | 3.36 | <0.01 |
| Cross-sectional | 17 | 1.12 (1.04-1.21) | 1.14 (1.02-1.27) | NS | 1.72 | <0.05 |
| Between types | 0.34 | NS | ||||
| By sex | ||||||
| Males | 7 | 1.29 (0.94-1.76) | 1.29 (0.94-1.76) | NS | 0.55 | NS |
| Females | 11 | 1.50 (1.25-1.81) | 1.59 (1.16-2.19) | NS | 2.40 | <0.01 |
| Both | 15 | 1.08 (1.01-1.16) | 1.10 (0.99-1.22) | NS | 1.81 | <0.05 |
| Between sexes | 5.57 | <0.01 | ||||
| By diagnosis | ||||||
| Mortalityi or hospitalisation | 11 | 1.37 (1.13-1.66) | 1.40 (1.12-1.74) | NS | 1.13 | NS |
| GOLD Stage 1+ | 10 | 1.09 (1.01-1.19) | 1.11 (0.97-1.27) | NS | 2.22 | <0.05 |
| Other | 12 | 1.15 (1.02-1.29) | 1.23 (0.97-1.56) | NS | 2.54 | <0.01 |
| Between diagnoses | 2.24 | NS | ||||
| By method for taking asthma into account | ||||||
| Included | 4 | 1.19 (1.00-1.41) | 1.19 (1.00-1.41) | NS | 0.45 | NS |
| Adjusted for | 4 | 0.94 (0.77-1.15) | 1.00 (0.72-1.39) | NS | 1.76 | NS |
| Excluded | 4 | 1.11 (0.99-1.26) | 1.24 (0.93-1.65) | NS | 3.63 | <0.05 |
| Ignored | 21 | 1.18 (1.08-1.30) | 1.27 (1.07-1.50) | NS | 2.08 | <0.01 |
| Between methods | 1.49 | NS | ||||
| By number of cases estimate is based on | ||||||
| <50 | 11 | 1.29 (0.97-1.71) | 1.26 (0.83-1.92) | NS | 1.85 | <0.05 |
| 50–149 | 9 | 1.62 (1.35-1.96) | 1.62 (1.35-1.96) | NS | 0.53 | NS |
| 150+ | 13 | 1.07 (1.00-1.15) | 1.08 (0.97-1.20) | NS | 1.97 | <0.05 |
| Between numbers | 8.65 | <0.001 | ||||
| By extent of confounder adjustment | ||||||
| Unadjusted for age | 6 | 1.11 (0.98-1.25) | 1.15 (0.94-1.42) | NS | 2.55 | <0.05 |
| Adj. for age+ <5 variables | 18 | 1.16 (1.05-1.28) | 1.26 (1.06-1.49) | NS | 1.75 | <0.05 |
| Adj. for age+ 5+ variables | 9 | 1.13 (1.00-1.27) | 1.20 (0.95-1.53) | NS | 2.61 | <0.01 |
| Between groups | 0.16 | NS | ||||
| By definition of never smoker | ||||||
| Never any product | 4 | 1.22 (0.93-1.61) | 1.28 (0.88-1.87) | NS | 1.34 | NS |
| Never, product unstated | 15 | 1.10 (1.01-1.20) | 1.16 (0.99-1.36) | NS | 2.15 | <0.01 |
| Other | 14 | 1.17 (1.06-1.30) | 1.25 (1.05-1.50) | NS | 2.18 | <0.01 |
| Between definitions | 0.63 | NS | ||||
| By definition of exposure | ||||||
| Spouse specifically | 7 | 1.26 (1.02-1.55) | 1.26 (1.02-1.55) | NS | 0.60 | NS |
| Other lives with smoker | 15 | 1.14 (1.02-1.29) | 1.31 (1.08-1.59) | <0.01 | 1.85 | <0.05 |
| Other | 11 | 1.11 (1.03-1.21) | 1.13 (0.95-1.34) | NS | 3.31 | <0.001 |
| Between definitions | 0.55 | NS | ||||
| All severer COPD | 7 | 1.29 (1.10-1.52) | 1.29 (1.10-1.52) | NS | 0.94 | NS |
a Definition of COPD as shown in the body of Table 1, severer COPD in footnotes to Table 1. Data as shown in Table 3
b Or nearest equivalent to spouse or household member (see text and Table 3)
c Heterogeneity relates to variation between studies within subgroup, except for results given in italics which relate to heterogeneity between subgroups
d N number of estimates in meta-analysis
e Egger test p expressed as <0.001, <0.01, <0.05, <0.1 or NS (p≥0.1)
f DF degrees of freedom
g p expressed as <0.001, <0.01, <0.05, <0.1 or NS (p≥0.1)
h Includes one study from Turkey and one from Lebanon
i Including study 22
| Study No. | Author | Typea | Sex | Exposure Source | Level | No. of cases | Odds ratio (95% CI)b | Trend pc |
|---|---|---|---|---|---|---|---|---|
| COPD: | ||||||||
| 3 | Hirayama | L15 | F | Husband | Never smoked Ex smoker or 1–19/day 20+/day | 28 65 37 | 1.00 1.29 (0.79-2.12)d 1.60 (0.92-2.78)d | NS |
| 6 | Kalandidi | CC | F | Husband | Never smoked Daily consumption ≤1 pack/day ≥1 pack/day Lifelong consumption ≤300,000 cigs 300,000+ cigs | 13 35 37 52 38 | 1.00 2.5 (1.3-5.0) 1.5 (0.8-2.7) 1.30 (0.64-2.64)d 1.70 (0.72-4.03)d | NS NS |
| 8 | Dayal | CS | M+F | Cohabitants | No smoker ≤1 pack/dayf >1 pack/dayf | 74e 76e 69e | 1.00 1.16 (0.78-1.72) 1.86 (1.21-2.86) | ++ |
| 10 | Enstrom | L39 | F M | Husband Wife | Per levelg Per levelg | 173 91 | 0.99 (0.92-1.06) 1.06 (0.91-1.25) | NS NS |
| 13 | McGhee | CC | M+F | Cohabitants | No smoker 1 smoker 2+ smokers | 84 54h | 1.00 1.85 (1.14-3.00) 2.51 (1.22-5.18) | ++ |
| 14 | Sezer | CC | F | Cohabitants | <10 years 10–19 years 20–29 years 30+ years | 13 12 20 29 | 1.00 1.19 (0.58-5.68) 2.46 (0.83-7.33) 4.96 (1.65-14.86) | ++ |
| 16 | Yin | CS | M+F | Cohabitants | No smoker 1 smoker 2+ smokers <2 years of 40 hours/wk 2–5 years of 40 hours/wk 5+ years of 40 hours/wk | 195 201 33 273 73 83 | 1.00 0.96 (0.77-1.20) 0.92 (0.62-1.36) 1.00 1.11 (0.84-1.47) 1.60 (1.23-2.10) | NS ++ |
| 18 | Wu | CC | F | Lifetime cohabitants and co- workers | No exposure <32 years 32+ years | 41 58 69 | 1.00 1.86 (1.10-3.17)d 2.53 (1.51-4.26)d | +i |
| 19 | Jordan | CS | M+F | Any exposure | Total No exposure 1–19 hours/wk 20+ hours/wk | 779e | 1.00 1.11 (0.94-1.31) 1.10 (0.81-1.49) | NS |
| 22 | He | L17 | M+F | Cohabitants and co- workers | Score 0f Score 1-2 Score 3-4 Score 5-6 | 10 8 13 5 | 1.00 1.52 (0.57-4.04) 2.32 (0.98-5.50) 5.01 (1.65-15.24) | ++ |
| 27 | Kim | CS | M+F | Home or workplace | Total No exposure ≤6 hours/day >6 hours/day | 323 | 1.00 0.83 (0.58-1.19) 1.75 (0.47-6.59) | NS |
| Severer COPD: | ||||||||
| 19 | Jordan | CS | M+F | Any exposure | Total No exposure 1-19 hours/wk 20+ hours/wk | 334e | 1.00 1.10 (0.81-1.49) 1.33 (0.74-2.38) | NS |
a Study types are CC = case-control, CS = cross-sectional, L = longitudinal. For longitudinal studies, number of years follow-up is shown
b RRs from longitudinal studies are taken as being equivalent to ORs
c NS p≥0.05, + p<0.05, ++ p<0.01
d OR and/or CI estimated from data provided
e Approximate estimates
f Sum of smoking levels for all cohabitants
g For husband smoking, there were 8 levels: never, former, current pipe/cigar, and current cigs/day 1-9, 10-19, 20, 21-39 and 40+. For wife smoking there were 7 levels, as for husband except with no level for pipe/cigar
h Number of cases is for the exposed groups combined
i Trend estimated from data provided
j Sum of scores for exposure at home (0 = no exposure, 1 = <4 pack years, 2 = 4 to <8 pack years, 3 = ≥8 pack years) and at work (0 = no exposure, 1 = <5, 2 = 5 to <15, 3 = ≥15, calculated from (pack years x smokers x hours/day)/100
From Table 3 it can be seen that, of the 33 individual OR estimates given for COPD, 24 are above 1.00, seven of these increases being significant at p<0.05. Eight studies reported an OR below 1.00, but only in study 4 for females was the reduction statistically significant. Study 25 reported an OR of 1.00, while study 24, excluded from the meta-analyses, did not present an OR but reported no significant relationship with duration or type of exposure. In addition, five studies presented a total of seven OR estimates for severer COPD, with five estimates above 1.00 (one significantly so and one marginally significant) and two non-significantly below 1.00.
Table 4 demonstrates that the overall evidence for the main exposure index shows some increased risk of COPD, with the random-effects OR, based on 33 independent estimates, being 1.20 (95% CI 1.08-1.34) with no clear evidence of publication bias (0.05<p<0.1), but clear heterogeneity (p<0.001). The largest contributors to this were the high ORs for studies 18 and 21 and in females for study 13 and the low OR in females for study 4.
Although there was no significant heterogeneity by continent, a significant increase was seen for North America (1.19, 1.01-1.41, n = 10) and Asia (1.34, 1.08-1.67, n = 10), but not for other locations. There was no significant heterogeneity by period of publication or study type. There was evidence of heterogeneity by sex (p<0.01), with a significant increase only for females (1.59, 1.16-2.19, n = 11). There was no heterogeneity by aspects of diagnosis, although the estimates were highest for definitions based on mortality or hospitalisation (1.40, 1.12-1.74, n = 11). However, there was significant heterogeneity (p<0.001) by numbers of cases, with larger ORs from studies of less than 50 cases (1.26, 0.83-1.92, n=11) and from studies of 50-149 cases (1.62, 1.35-1.96, n=9) than for studies of 150 or more cases (1.08, 0.97-1.20, n=13). There was no significant evidence of heterogeneity by extent of confounder adjustment, or by how never smokers or the exposure index were defined. For all these subgroup analyses, there was little evidence of publication bias, but evidence of heterogeneity in some subgroups.
The combined OR for severer COPD was significant (1.29, 1.10-1.52, n=7).
There was also evidence of a dose-response relationship, as shown in Table 5, with six of 11 studies investigating this reporting a statistically significant positive trend. Study 16 reported no trend in relation to the number of smokers in the household, but did report positive dose-response relationships for years of ETS exposure at home and at work. Study 19, which found no relationship with the main COPD outcome, also presented dose-response relationships for severer COPD, again finding no significant increase in risk with increasing exposure.
Five studies also presented additional results for other indices of ETS exposure, as shown in Table 6. Four studies (16, 22, 23, 26) looked at exposure at work, all but study 23 also presenting results for combined exposure at home and at work. Study 5 produced a combined index of adulthood exposure at home or work, or during travel or leisure. Three studies (16, 23, 26) considered childhood ETS exposure, study 23 studying exposure from both the mother and the father, and also looking at parental smoking during pregnancy.
| Study No. | Author | Typea | Sex | Number of cases | Index of exposureb | Odds ratio (95% CI)c | Meta- analysisd | |
|---|---|---|---|---|---|---|---|---|
| Unexposed | Exposed | |||||||
| COPD: | ||||||||
| 5 | Lee | CC | F | 7 | 5 | Combined index of adulthood exposure at home, work, during travel and leisuree | 1.04 (0.34-3.20)f | A |
| M | 1 | 3 | 1.18 (0.19-7.42)f | A | ||||
| 16 | Yin | CS | M+F | 225 | 204 | Childhood – home | 0.87 (0.71-1.06)f | C |
| 240 | 187 | Adulthood – work | 0.96 (0.78-1.19)f | W | ||||
| 191 | 238 | Adulthood - home and/or workg | 1.24 (1.01-1.51)f | A | ||||
| 22 | He | L17 | M+F | 10 | 26 | Workplaceh | 2.52 (1.00-6.38) | W |
| M | 8 | 15 | Adulthood – home and/or work | 2.15 (0.86-5.39) | A | |||
| F | 2 | 11 | 3.31 (0.69-15.82) | A | ||||
| 23 | Waked | CS | M+F | Total 25 | During pregnancy: mother | 1.59 (0.51-4.92)f | ||
| During pregnancy: father | 1.69 (0.73-3.90)f | |||||||
| Childhood: mother | 1.17 (0.39-3.52)f | C | ||||||
| Childhood: father | 1.36 (0.61-3.07)f | |||||||
| Workplace | 0.75 (0.18-3.14)f | W | ||||||
| 26 | Hagstad | CS | M+F | 25 | 78 | Previous workplacei | 1.42 (0.86-2.33) | |
| 25 | 14 | Current workplacei | 1.17 (0.58-2.36) | W | ||||
| 25 | 10 | Previous and current workplacei | 1.74 (0.77-3.91) | |||||
| 25 | 5 | Ever at home and both previous and current workplacei | 3.80 (1.29-11.20) | |||||
| 25 | 3 | Currently at home and both previous and current workplacei | 5.73 (1.46-22.50) | |||||
| 25 | 115 | Ever at home and/or worki | 1.87 (1.20-2.91)f | A | ||||
| Severer COPD: | ||||||||
| 26 | Hagstad | CS | M+F | Total 27 | Childhoodj | 0.62 (0.28-1.35)f | ||
a Study types are CC = case control, CS = cross-sectional, L = longitudinal. For longitudinal studies, number of years follow-up is shown
b Comparison is with no exposure of the type specified, except where indicated otherwise
c RRs from longitudinal studies are taken as being equivalent to ORs
d A = Any adult, C = Childhood and W = Workplace indicate estimates included in Table 7 meta-analysis
e Comparison is with those with no exposure of any of the four types, or at most little exposure from one of them
f OR and/or CI estimated from data provided
g Comparison is with those with <2 years of 40 hours per week exposure
h Compares exposed at work only to unexposed. Excludes those exposed at home
i Comparison group is subjects not exposed to ETS from any source
j From 65
The ORs for these other exposure indices are supported by Figure 3 (workplace) and Figure 4 (overall adult), while Table 7 presents the results of meta-analyses. Note that Figure 4, and the meta-analyses for overall adult exposure, consider not only the ORs indicated in Table 6, but also include estimates from Table 3 for those ten studies (4, 11, 12, 15, 17, 18, 19, 21, 25, 27) for which the exposure was at least from home and work.
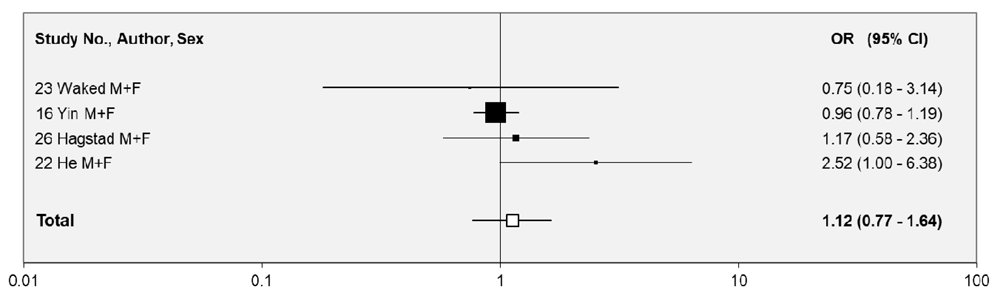
Individual study estimates of the OR and its 95%CI are shown sorted in increasing order of OR. These are shown as numbers, and also graphically on a logarithmic scale. Random-effects estimates of ORs and 95%CIs are also shown. Studies are identified by the study number shown in Table 1. In the graphical representation, ORs are indicated by a square, with the area of the square proportional to the weight.
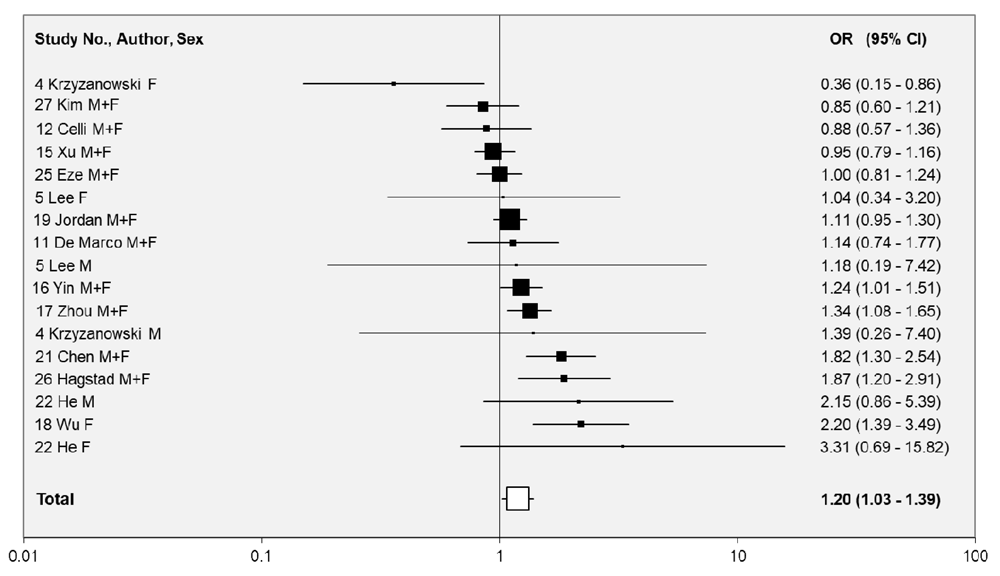
Individual study estimates of the OR and its 95%CI are shown sorted in increasing order of OR. These are shown as numbers, and also graphically on a logarithmic scale. Random-effects estimates of ORs and 95%CIs are also shown. Studies are identified by the study number shown in Table 1. In the graphical representation, ORs are indicated by a square, with the area of the square proportional to the weight.
| Fixed-effect | Random-effects | Publication bias | Heterogeneity | |||
|---|---|---|---|---|---|---|
| Index of exposure | Nb | Odds ratio (95% CI) | Odds ratio (95% CI) | pc | Chi squared per DFd | pe |
| Workplace | 4 | 1.01 (0.83-1.23) | 1.12 (0.77-1.64) | NS | 1.43 | NS |
| Overall adultf | 17 | 1.16 (1.07-1.25) | 1.20 (1.03-1.39) | NS | 2.65 | <0.001 |
| Childg | 2 | 0.88 (0.72-1.07) | 0.88 (0.72-1.07) | - | 0.27 | NS |
a Definition of COPD as shown in the body of Table 1. Data as shown in Table 6 excluding severer COPD
b Number of estimates in meta-analysis
c Egger test p expressed as <0.001, <0.01, <0.05, <0.1 or NS (p≥0.1)
d Degrees of freedom
e p expressed as <0.001, <0.01, <0.05, <0.1 or NS (p≥0.1)
f Index includes “home or workplace” or combined index of any adulthood exposure. Note that this meta-analysis not only includes those estimates marked with an A in Table 6, but also includes estimates from Table 3 for studies 4, 11, 12, 15, 17, 18, 19, 21, 25 and 27
g Preferring exposure from the mother in study 23. Estimates would be 0.89 (0.74-1.08) fixed and 0.91 (0.70-1.18) random, preferring exposure from the father
Of the four ORs included in the meta-analysis of COPD for exposure at work, two were above 1.00, one of borderline statistical significance, and two were below 1.00, the combined estimate being 1.12 (0.77-1.64). Note that in study 26 there was a choice of workplace OR estimates, with the meta-analysis including that for current exposure. Using estimates for previous or ever exposure would not have affected the conclusion that there was no clear relationship of COPD to workplace ETS exposure.
Of the 17 ORs included in the meta-analysis for overall adult exposure, 12 were above 1.00, five significantly so, with one equal to 1.00, and four less than 1.00. The combined estimate of 1.20 (1.03-1.39) was also significantly increased.
There was no clear association of COPD with childhood ETS exposure, with none of the ORs shown in Table 6 being significant. Only two estimates could be included in the meta-analysis, giving an overall estimate of 0.88 (0.72-1.07).
There was no significant evidence of publication bias for workplace or adult exposure, the data being too limited to assess this for childhood exposure. However, there was evidence of heterogeneity (p<0.01) for overall adult ETS exposure.
The limited further dose-response data shown in Table 8 added little to the data already shown in Table 5.
| Study No. | Author | Typea | Sex | Exposure | No. of cases | Odds ratio (95% CI) | Trend pb | |
|---|---|---|---|---|---|---|---|---|
| Source | Level | |||||||
| 5 | Lee | CC | F | Combined index of adulthood exposure at home, work, during travel and leisure | Score 0-1c | 7 | 1.00 | |
| Score 2-4 | 4 | 1.05 (0.29-3.75) | ||||||
| Score 5-12 | 1 | 1.03 (0.12-8.85) | NSd | |||||
| M | Score 0-1 | 1 | 1.00 | |||||
| Score 2-4 | 2 | 0.83 (0.07-9.56) | ||||||
| Score 5-12 | 1 | 1.90 (0.11-32.61) | NSd | |||||
| 16 | Yin | CS | M+F | Childhood | No smoker | 225 | 1.00 | |
| 1 smoker | 157 | 0.89 (0.72-1.10) | ||||||
| 2+ smokers | 47 | 0.81 (0.58-1.12) | NS | |||||
| Co-workers | No smoker | 240 | 1.00 | |||||
| 1 smoker | 15 | 0.88 (0.51-1.52) | ||||||
| 2+ smokers | 172 | 0.97 (0.78-1.20) | NS | |||||
| <2 years of 40 hours/wk | 286 | 1.00 | ||||||
| 2–5 years of 40 hours/wk | 65 | 1.35 (1.01-1.80) | ||||||
| 5+ years of 40 hours/wk | 78 | 1.50 (1.14-1.97) | ++ | |||||
| Cohabitants and co-workers | <2 years of 40 hours/wk | 191 | 1.00 | |||||
| 2–5 years of 40 hours/wk | 82 | 0.95 (0.72-1.24) | ||||||
| 5+ years of 40 hours/wk | 156 | 1.48 (1.18-1.85) | ++ |
We rejected papers for various appropriate reasons. These included the following: failing to give results for an endpoint equivalent to COPD; giving results only for COPD exacerbation or prognosis; not presenting results for never smokers; describing studies without a control group; not presenting results for those unexposed to ETS; and presenting less useful results than reported in another publication.
Twenty-eight epidemiological studies did qualify for inclusion, and from 33 estimates of the risk of COPD associated with ever having a spouse who smoked, or the nearest equivalent ETS exposure index available, random-effects meta-analysis gave a significantly increased OR estimate of 1.20 (1.08-1.34). There was also some evidence of dose-response. While the clear relationship of smoking with COPD31 makes it plausible that some effect will also be evident for ETS, one must emphasize that exposure is much less than from active smoking, as noted in the Introduction. Also, various limitations of the evidence, discussed below, make it difficult to estimate reliably the true extent of any causal relationship. However, one should also take into account the evidence of a relationship between ETS and wheezing46,66, a symptom of COPD.
Though four studies involved more than 500 cases, with the maximum 1097 in study 15, as many as ten of the 28 studies involved less than 100 cases, the quite small number of cases making it difficult to detect potential effects reliably.
The observation that ORs are only modestly raised for studies with larger numbers of cases but are greater for smaller studies suggests the possibility of publication bias, with authors being more likely to report stronger relationships. However formal tests for publication bias54 showed no clear evidence of its existence. One must note, though, that various large longitudinal studies, e.g. 67–70, reported results relating ETS to smoking-related diseases such as lung cancer or heart disease, but did not do so for COPD. If any relationship had been seen, these studies might well have been reported.
No study validated the lifelong non-smoking status of their participants, although study 18 did verify current active and passive exposure in a random sample of participants by measuring urinary cotinine levels. As some current and past smokers deny smoking when interviewed71, and as the smoking habits of spouses or household members are clearly correlated47, misclassification of even a few ever smokers as never smokers can cause relevant bias72, especially when, as is the case with COPD, the association with smoking is strong31.
All the longitudinal studies considered involved follow-up for at least 12 years. Of the five studies, three (studies 3, 7 and 10) assumed spousal smoking was unchanged during follow-up, only studies 4 and 22 collecting information on smoking status at multiple time points.
All these studies only considered COPD deaths which occurred in the original study area.
Although three case-control studies used population controls, the remaining three used control groups unlikely to be representative of the population from which the cases derived. Studies 6 and 14 used visitors to the hospital attended by the cases, and study 13 used as a control a person identified by the informant of a death as a “living person about the same age who was well known to the informant”, the informant then being asked about the lifestyle 10 years earlier of both decedent and control.
Over half of the studies were of cross-sectional design, a design limited by difficulties in determining whether ETS exposure or disease onset occurred first.
As noted above, some studies made little or no adjustment for variables likely to differ between smoking and non-smoking households. Though ORs for the main exposure index did not vary significantly by extent of adjustment, it should be noted that adjustment for dietary variables and education explains a substantial part of the association of lung cancer with spousal smoking45. The same may be the case for COPD.
Definitions of COPD used were all consistent with the inclusion criteria. However, they still varied somewhat between study, further adding uncertainty to the meta-analysis results. Even given the inclusion criteria, there are doubts about the appropriateness of the diagnostic criteria used in some studies. In study 8, for example, the definition included asthma as well as chronic bronchitis and emphysema, the diagnosis being reported by the head of the household, and not necessarily made by a doctor.
While random errors in determining ETS exposure led to underestimation of the relationship of COPD with ETS, errors may not be random. Twenty-three of the 28 studies considered were of case-control or cross-sectional design, where recall bias may exist if those with COPD tend to overestimate their ETS exposure compared to those without COPD. Exposure was generally not validated by biochemical markers or air measurements taken at home.
Only 15 studies (4, 5, 11, 12, 15–19, 21–23, 25–27) provided data on ETS exposure from sources other than the home. Five (4, 12, 17, 18, 27) presented results only for a combined household and workplace exposure index, with a further five (11, 15, 19, 21, 25) only presenting results for total exposure irrespective of location, results used in our analyses as the nearest equivalent which was available to smoking by the spouse or household member. While there are far less available data on risk of COPD from ETS exposure specifically in the workplace or in childhood than on smoking in the home, the available data show no clear relationship of risk with these less studied exposure indices.
A review in 200773 considered that “ETS exposure may be an important cause of COPD”. However, this conclusion was based on only six studies, one examining absolute risk of COPD in relation to changes in tobacco consumption, and one comparing lung function of employees in bars and restaurants before and after a smoking ban. Also it seemed that at least some of the others considered were not restricted to never smokers.
A review in 201060 meta-analysed results from 12 studies and gave an overall estimate of 1.56 (1.40-1.74), somewhat higher than our estimate. Not all of the studies included were of COPD, some being based on chronic bronchitis symptoms. Also, some studies were based on current non-smokers rather than on lifelong never smokers.
In 2013, Bentayeb et al.61 reviewed evidence on indoor air pollution and respiratory health in those aged over 65 years. After considering 33 papers (only one16 presenting relevant results on ETS and COPD risk in non-smokers), they reported that the most consistent relationship found was between ETS exposure and COPD risk. However, the findings did not allow causal inference due to heterogeneity of the studies considered, measurement errors in exposure assessment, variable outcome definition, and lack of information on lifetime exposure to air pollution. The authors concluded that more investigations are needed to understand the relationship of indoor air pollution to respiratory health in the elderly.
A review in 201474 reached similar conclusions, the authors stating that “second-hand exposure to tobacco smoke has also been shown to be associated with the risk of COPD, although more robust evidence needs to be generated”. These conclusions were derived from only eight studies, some concerned with respiratory symptoms rather than COPD. Also, one study did not restrict any analyses to never smokers.
A review in 201562 included only five studies in the meta-analyses. The estimated risk of COPD in ETS-exposed participants was higher than we estimated, being 1.66 (1.38-2.00) for both sexes combined, 1.50 (0.96-2.28) for males and 2.17 (1.48-3.18) for females. However, these estimates were based on, respectively, three, one and one estimates, the authors examining three further studies but not including them in their meta-analysis due to low study quality. However, two of the studies they did include were not based on lifelong never smokers. Many other studies that might have been included were not. The authors noted that “the few existing studies on second-hand smoke exposure and COPD differ considerably, although the results indicate a positive association” and that “further research is needed, to provide more adequate primary studies which account for confounding and other biases”.
A review in 201663 of “the effects of smoking on respiratory health” also considered effects of ETS exposure. However, only three studies were cited, two not satisfying our inclusion criteria. Noting the variability in the results, the authors only pointed to the need for additional studies.
Generally these reviews point to an association between ETS exposure and risk of COPD without concluding that a causal relationship has clearly been established. The present review, which includes far more studies, confirms the association and provides evidence that is strongly suggestive of a true effect. While this suggestion is not inconsistent with the view of the Global Burden of Disease Study 201775 that second-hand smoke is a risk factor for COPD, limitations of the evidence, discussed above, precludes a more definitive conclusion.
In response to a comment from a reviewer (Dr Maio), we updated our searches by a further two years. While this identified an additional 99 publications, only one76 satisfied our inclusion criteria. That paper reported age and sex adjusted hazard ratio estimates by level of ETS exposure, which, when combined, gave an exposed/unexposed estimate of 2.25 (95% CI 1.05-4.82). Including this estimate, based on only 33 COPD cases, had little effect on the meta-analysis results shown in Table 4. Thus, the overall random-effects estimate of 1.20 (95% CI 1.08-1.34) for all COPD was changed only to 1.22 (1.09-1.36), while that for Asia was changed only from 1.34 (1.08-1.67) to 1.38 (1.11-1.72).
Taken in conjunction with the strong association of smoking with COPD, the significant relationship seen for the main index of ETS exposure, and the evidence of a dose-response relationship is highly suggestive that ETS also increases risk of COPD. However, the absence of well-designed and fully reported large studies, and the limitations noted above make it difficult to obtain an accurate estimate of the true magnitude of any possible effect. More definitive studies are required to reach a firmer conclusion.
Supplementary file 1: Rejected studies: In preparing the tables and figures in this document, certain papers that might be thought to provide relevant data have not been referred to. For each of these papers, this appendix notes the authors, date of publication and country and the reasons for not referring to them. https://doi.org/10.17605/OSF.IO/8APGK77
Supplementary file 2: PRISMA checklist
https://doi.org/10.17605/OSF.IO/8APGK77
Supplementary file 3: Forest plots in Excel
We thank Japan Tobacco International S.A. for supporting the work described. The opinions and conclusions of the authors are their own, and do not necessarily reflect the position of Japan Tobacco International S.A. We also thank Diana Morris and Yvonne Cooper for assistance in typing various drafts of the paper and obtaining relevant literature, and all the researchers who published the reports which formed the basis of our work. Finally, we thank the reviewers for their helpful comments, and the time they spent.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Haahtela T, von Hertzen L, Anto JM, Bai C, et al.: Helsinki by nature: The Nature Step to Respiratory Health.Clin Transl Allergy. 2019; 9: 57 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Passive smoking, Chronic Respiratory Diseases, impact of conflict
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 09 Jan 20 |
read | |
|
Version 2 (revision) 19 Dec 19 |
read | read |
|
Version 1 05 Feb 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)