Keywords
Transmembrane transport, ion channels, passive transport, active transport, rectification, bidirectional assymetric flow, AMPA-Kainate receptor, excitable cell
Transmembrane transport, ion channels, passive transport, active transport, rectification, bidirectional assymetric flow, AMPA-Kainate receptor, excitable cell
The new version contains edits and comments that take into account the observations made by Reviewers 1 and 2, which can be found in the open review section. The most important changes include (i) expanded the Introduction section with details about the transmembrane transport, (ii) precisions about, and notation additions to the formulas for the energy associated to ATP hydrolysis, (iii) an expansion of the Discussion section about the sharpness of the upstroke in the action potential in neurons. Header lines with physical units have been added to the data files for more clarity. Also, a JuPyTeR notebook has been included as Supplementary File 3. The notebook contains code to generate the figures for the paper.
See the author's detailed response to the review by Kyle C.A. Wedgwood
See the author's detailed response to the review by Moisés Santillán
One of the most important physiological mechanisms underlying communication within and between cells is the transport of molecules across membranes. Molecules can cross membranes either passively (Stein & Litman, 2014), or via active transport (Bennett, 1956). Molecules are passively transported across a membrane when they move along their (electro)chemical gradient. In contrast, active transport involves transmembrane motion of molecules against their electrochemical gradients. One important functional distinction between channels and pumps is that the rate of transport for channels is generally several orders of magnitude faster than the rate for pump-mediated transport (Gadsby, 2009; Ussing, 1949a). Such differences are reflected in the sizes of different transmembrane fluxes typically observed in excitable cells (Herrera-Valdez & Lega, 2011).
Passive transport may occur through transmembrane proteins (Hille, 1992; Stein & Litman, 2014) that may be selective for molecules of specific types (Almers & McCleskey, 1984; Doyle et al., 1998; Favre et al., 1996), typically mediating (electro)diffusion through them. Sometimes these proteins are also gated by conformational changes triggered by different signlling mechanisms. Passive transport has also been observed in pores spontaneously formed within synthetic lipid bilayers (Blicher & Heimburg, 2013), which could also occur in natural conditions (Gurtovenko & Anwar, 2007). One example of importance in the context of energy homeostasis is the transport of monosaccharides and other similar molecules through GLUT transporters. GLUT transporters first bind to their substrates, triggering a conformational change that allows the substrate to cross in the direction of its chemical gradient (e.g. GLUT5 is highly specific to fructose, Mueckler & Thorens (2013)). Channels are another important class of transmembrane proteins that typically mediate fast passive transport, often displaying selectivity for specific ion types, gated by changes in the membrane potential (Covarrubias et al., 1991; Peng & Wu, 2007) or the binding of a ligand molecule (e.g. AMPA-Kainate synaptic receptors, Bowie (2002)).
Active transport is mediated by transmembrane proteins commonly called pumps, or carriers, that mechanically translocate the molecules they transport (Bennett, 1956; Ussing, 1949b; Ussing, 1949c). The energy for primary active transport is usually obtained from biochemical reactions (e.g. ATPases, light-driven pumps). For instance, the energy to transport molecules against their (electro)chemical gradient via ATPases is obtained from hydrolysis of ATP (Chapman, 1973). In secondary and tertiary active transport, the electrochemical gradient of at least one the ion types provides the energy to transport other molecules against their (electro)chemical gradient (SKou, 1965). Two large classes of non-primary active transport pumps are the symporters and counterporters, carrying at least two types of molecules in the same, or in opposite directions, respectively, with at least one type against its electrochemical gradient. For instance Na-H exchangers carry Na+ and H+ in opposite directions, typically using the driving force from Na+. In contrast, K-Cl symporters carry K+ and Cl− in the same direction, which means that one of the two ion types is carried against its concentration gradient. This is because the K+ and Cl− concentration gradients are typically oriented in opposite directions. As a consequence, the movement of one of the two ions releases energy from its electrochemical gradient, enabling the transport of the other ion against its gradient.
Theoretical models of transmembrane transport play a critical role in developing our understanding of the function and mechanisms underlying electrical signaling and cellular excitability (Barr, 1965; Cole, 1965; DiFrancesco & Noble, 1985; Endresen et al., 2000; Gadsby, 2009; Goldman, 1943; Kell, 1979; Kimizuka & Koketsu, 1964; Läuger, 1973; Stevens & Tsien, 1979; Wiggins, 1985a; Wiggins, 1985b; Wiggins, 1985c), and some of its associated pathologies (Ashcroft, 2005; Marbán, 2002). The best known transmembrane transport models include the widely used conductance-based formulation from the seminal work of Hodgkin & Huxley (1952), the Goldman-Hodgkin-Katz equation (Goldman, 1943; Hodgkin & Katz, 1949; Pickard, 1976), and several other expressions for carrier and channel mediated transport with many different functional forms (DiFrancesco & Noble, 1985; Rasmusson et al., 1990a; Rasmusson et al., 1990b; Rosenberg & Wilbrandt, 1955). Other formulations for ionic transport across membranes derived from biophysical principles available in the literature include those in the work by Jacquez & Schultz (1974); Kimizuka & Koketsu (1964); Pickard, 1969; Pickard, 1976. See also Jacquez (1981) and similar work by Endresen et al. (2000), and those in the excellent book by Johnston et al. (1995). Such formulations describe the relationship between the activity and permeability of ions across membranes, and the transmembrane potential. However general models that describe physiological transport including passive and active transport of charged or non-charged molecules, with bidirectional but possibly asymmetric flows, are still missing. The work presented here builds upon the results previously mentioned by describing transport macroscopically in terms of the energy required to move molecules across a membrane. The result is a general formulation with a common functional form for both passive and active transport (Herrera-Valdez, 2014) that also includes a term that regulates the asymmetry in the flow (rectification). The details of the derivation and examples of fits to experimental data and features like asymmetric bidirectional flow can be found in the next section. An application of the general formulation is illustrated with models for the transmembrane potential dynamics in cardiac pacemaker cells and striatal fast spiking interneurons (Supplementary File 1). A derivation and connection of the general formula with existing formulations like the Goldman expression for current can be in the Supplementary File 1.
An earlier version of this article is available on PeerJ as a preprint https://doi.org/10.7287/peerj.preprints.1312v8.
Consider a system consisting of a biological membrane surrounded by two aqueous compartments (e.g. extracellular and intracellular). Assume, to start with, that the compartments contain molecules of a single type s (e.g. Na+, K+, glucose), possibly in different concentrations. Let ∆Gs be the energy required for the transport of the molecules across the membrane in a specific direction (e.g. inside to outside). To write an expression for ∆Gs it is necessary to take the direction of motion of the s-molecules into account. To do so, label the extracellular and intracellular compartments as 0 and 1, respectively, and let cs and ds ∈ {0, 1} represent the source and the destination compartments for the transport of the s-molecules. The pair (cs, ds )=(0,1) represents inward transport and the pair (cs, ds )=(1,0) represents outward transport. The work required to transport ns molecules of type s from compartment cs to compartment ds can then be written as
(Aidley, 1998; Blaustein et al., 2004; De Weer et al., 1988) where q, zs, [s]0, and [s]1 represent the elementary charge, the valence, the extracellular, and the intracellular concentrations for the molecules of type s, respectively. The variable v represents the transmembrane potential. Two particular cases are worth noticing. First, if s is an ion, then zs ≠ 0 and Equation (1) becomes
where vs is the Nernst potential for the s-molecules1 (Nernst, 1888). Second, if the s-molecules are not charged, then zs = 0 and the work required to move the s-type molecules from cs to ds simplifies to
If ∆Gs < 0, then the molecules can be transported passively (e.g. electrodiffusion), decreasing the electrochemical gradient for s across the membrane. In contrast, if ∆Gs > 0, the transmembrane transport of s from cs to ds is not thermodynamically favorable, which means the transport from cs to ds requires energy that is not available in the electrochemical gradient for s (active transport). As a consequence, active transport of s would increase the driving force for the motion of s across the membrane.
To find an expression that describes a more general transport mechanism, assume that transport takes place as single events in which molecules of m different types move in parallel, or possibly sequentially (e.g. first Na+, then K+), across the membrane. Let S be a set that represents the types of molecules that are jointly transported in a single event. For instance, for Na+-H+ exchangers, S = {Na+, H+}, with m = 2. The energy required to transport these molecules is the sum of the energies required to transport each of the molecules in S. In other words,
As before, transport is thermodynamically favorable when ∆GS ≤ 0. If not, extra energy is required. To distinguish between these two cases, define the total energy ∆G of the transport mechanism, possibly including an extra source of energy, as
where δExt = 1 if ∆GS > 0, and 0 otherwise. If ∆GS > 0, then the energy from ATP hydrolysis or any other sources represented by δExt∆GExt should be negative and be larger size in comparison to ∆GS, so that ∆G ≤ 0, making the transport thermodynamically possible. In particular, for ATP-driven transport, the extra energy supplied by hydrolysis of ATP (Tanford, 1981; De Weer et al., 1988) is
with [ATP]1, [ADP]1, and [Pi]1 representing, respectively, the intracellular concentrations of adenosine triphosphate (ATP), adenosine diphosphate (ADP), and inorganic phosphate (Pi) (De Weer et al., 1988). The potential vATP ≈ −450 mV (Endresen et al., 2000), but could vary depending on the intracellular amounts of ATP, ADP, and Pi (De Weer et al., 1988). The first term in Equation (6) represents the "standard" change in Gibbs free energy for the hydrolysis of ATP2 Similar expressions could be derived for active transport driven by light, or other sources of energy. The concentrations of ATP, ADP, and Pi are assumed to be constant in most models presented here, but it should be noted that such concentrations are not necessarily constant, and in fact, may vary a lot in some cases, as it is the case for skeletal muscle (Wackerhage et al., 1998).
The formulation in Equation (5) can be combined with Equation (1) to derive a generalized expression for flux and model different known mechanisms of physiological transmembrane transport, possibly combining the transport of different molecules simultaneously (e.g. Na-H exchange). In this case, the forward direction of the transport would be described by the combined forward transport of each of the different molecules under consideration. For instance, the source and target compartments for Na+ and Ca2+ are different in Na-Ca exchangers. The stoichiometry for the transport mediated by Na-Ca exchangers in the forward direction involves three Na+ molecules moving inward (along their electrochemical gradient) in exchange for one Ca2+ molecule moving outward (against their electrochemical gradient) (Mullins, 1979; Venetucci et al., 2007).
Let α and β be the flux rates in the forward and backward directions, in units of molecules per ms per µm−2. These rates depend, a priori, on the energy required for the transport of the molecules in S. The net flux rate associated to the net transmembrane transport, can then be written as
How do α and β depend on ∆G? The steady state relationship between the energy ∆G and the the forward and backward flow rates, hereby represented by α and β, can be written as
where k is Boltzmann’s constant, and T the absolute temperature.
Assuming that α and β are continuous functions, the rates α and β can be rewritten as
where r and b represent the rate at which the transport takes place (molecules per ms per µm−2) and the bias of the transport in one of the two directions (b = 1/2 means the transport is symmetrical relative to the point at which ∆G=0). Note that the functional form of the α and β in Equations (9) are similar to those by Butler (1924); Erdey-Grúz & Volmer (1930). Also, notice that the steady state relationship between α and β in Equation (8) can be obtained from Equations (9), for any r and any b. However, it should be the case that r and b vary in specific ranges depending on the physico-chemical characteristics of the pore through which molecules cross the membrane, and in general, on the transport mechanism. As already mentioned, the rate r should be larger for electrodiffusive transport mediated by ion channels, in comparison to the slower transport rates for facilitated diffusion and active transport meditated by carrier proteins and pumps. The rate r may depend on temperature (Sen & Widdas, 1962), the transmembrane potential (Starace et al., 1997), the concentrations inside and outside of the membrane (Yue et al., 1990), and other factors (Novák & Tyson, 2008). If the parameter b ∈ [0, 1], then b∆G and (b − 1)∆G have opposite signs and can be thought of as the energies required to the transport of the molecules in S in the forward and backward directions, respectively, with b biasing the transport in the forward direction when close to 1, and in the backward direction when close to 0 (Figure 1).
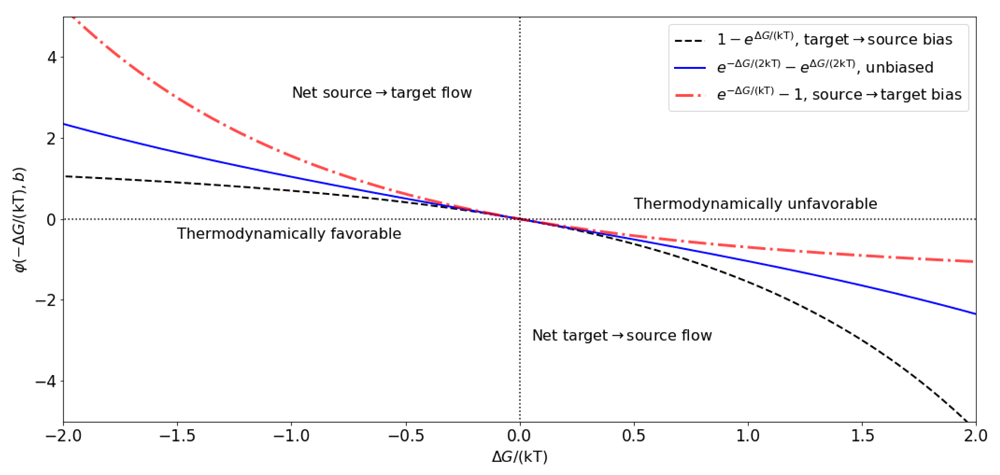
The energy ∆GS necessary can be found on the right axis if the transport is primary active, and on the left axis when transport is secondary active or passive. Extra energy from ATP hydrolysis or other sources added to ∆GS shift the total energy to the left axis and make the transport thermodynamically possible. See Table 1 for examples.
The flux can then be written explicitly combining Equation (7) and Equation (9) to obtain,
Taking the above observations into account, it is possible to combine Equation (4) and Equation (5), to write an expression similar to Equation (8) for the steady state balance between the forward and backward transport of all the molecules in S.
Substitution of the formulas for ∆G from Equation (4) Equation (5) into Equation (10), the flux rate resulting from simultaneously transporting molecules in S across the membrane can be written explicitly
where vT = kT/q and
represents the net number of charges moved across the membrane. Note that vExt should be vATP for ATPases. The first, more complex, form of the flux in Equation (11) could be useful when working with models for which changes in the concentrations of different molecules are relevant.
If the transport is electrogenic, then the product qη (in Coulombs) represents the net charge moved across the membrane, relative to the extracellular compartment. Non electrogenic transport yields η = 0, which means the flow does not depend on the transmembrane potential, and
If only ions are involved in the transport, the flux simplifies to
where
The quantity vo/η can be thought of as a reversal potential. If η < 0, then positive charge is transported inward, or negative charge is transported outward. In contrast, η > 0 means that positive charge is transported outward or negative charge transported inward. For instance, inward electrodiffusion of single Na+ ions gives an η = −1, which can be thought of as loosing one positive charge from the extracellular compartment in each transport event (see Table 1).
A list with examples of energies and total charge movements for different transport mechanisms can be found in Table 1.
Transmembrane current. The flux that results in electrogenic transport (Equation (11) and Equation (14)) can be converted to current density after multiplication by qη. In short form,
with qr in Amperes/m2 or equivalent units.
Substitution of Equation (11) or Equation (14) into Equation (16) yields a general formula for the current generated by transmembrane ionic flux (Figure 2), that uses the same functional form for channels (protein or lipid) and pumps. Recall that Equation (16) can also be written explicitly in terms of the transmembrane concentrations of one or more of the ions involved using Equation (11). It is possible to derive expressions for r that take into account biophysical variables like temperature and the shape and length of the pore through which the molecules cross (Endresen et al., 2000; Pickard, 1969).
A number of nontrivial and important properties of transmembrane ionic currents, including rectification, are also described by Equation (16). Also, different models for current already in the literature can be obtained by making approximations or setting particular cases from Equation (16). Examples include electrodiffusive currents that result from integration of the Nernst-Planck equation along the length of membrane pore (Jacquez & Schultz, 1974; Johnston et al., 1995; Pickard, 1969). Of particular interest, conductance-based currents are linear approximations of the formulation (16), around the reversal potential for the current.
Lower order approximations to the general formulation and conductance based models. Conductance-based currents (Hodgkin & Huxley, 1952) are linear approximations of the general current from Equation (16), around the reversal potential vo/η. To see this, use Taylor’s theorem (Courant & John, 2012; Spivak, 2018) to rewrite the general current from Equation (16) as a series around vo
Truncation of the series to first order gives
where g = η2qr/vT is in units of nS/µm2, which has the functional form of the conductance-based current used in the Hodgkin & Huxley (1952) model. For instance, the linear approximation for the current through an open sodium channels around vNa in Equation (18) gives gNa = qrNa/vT, and vo = ηNavNa, with ηNa = −1, so that iNa ≈ gNa(v − vNa).
Notice that third order approximations to Equation (14) can also capture rectification. In contrast, first order approximations (conductance-based models) cannot capture rectification.
Rectification results from asymmetric bidirectional flow. The flux of molecules across the membrane can be biased in either the outward or the inward direction when mediated by proteins. This was first called ”anomalous rectification” by Katz (1949), who noticed that K+ flows through muscle membranes more easily in the inward, than in the outward direction (Armstrong & Binstock, 1965; Adrian, 1969). It was later found that some K+ channels display the bias in the opposite direction (Woodbury, 1971). The former type of K+ current rectification is called inward, and the latter outward.
Rectification is a bias in either of the two directions of transport, which may result from changes in the structure of the proteins or pores through which the molecules cross the membrane (Hollmann et al., 1991; Riedelsberger et al., 2015). The type of rectification (inward or outward) depends on what molecules are being transported and on the structure of the proteins mediating the transport. Rectification is therefore not only a property of ions, as shown by molecules like glucose, which may cross membranes via GLUT transporters bidirectionally, but asymmetrically, even when the glucose concentration is balanced across the membrane (Lowe & Walmsley, 1986).
Rectification can be described by Equation (11) by setting b to values different from 1/2, and becomes more pronounced as b is closer to either 0 or 1. These values represent biases in the transport toward the source, or the target compartment, respectively. As a consequence, rectification yields an asymmetry in the graph of α − β as a function of ∆G (Figure 1). For electrogenic transport, rectification can be thought of as an asymmetric relationship between current flow and voltage, with respect to the reversal potential vo. The particular case b = 1/2 (non rectifying) yields a functional form for current similar to that proposed by Pickard (1969), and later reproduced by (Endresen et al., 2000), namely
From here on, subscripts will be used to represent different transport mechanisms. For instance, the current for a Na-Ca pump will be written as iNaCa.
Electrodiffusion of K+ through channels (η = 1 and vo = vK), is outward for v > vK, and inward for v < vK. The K+ current through the open pore is therefore
Current flow through inward rectifier channels (Riedelsberger et al., 2015) can be fit to values of bK < 1/2. For instance,
describes a current with limited flow of K+ in the outward direction, similar to the currents described originally by Katz (1949). Analogously, bK > 1/2 limits the inward flow. For example, the current
describes outward rectification (Riedelsberger et al., 2015).
Based on the work of Riedelsberger et al. (2015) on K+ channels, inward (outward) rectification arises when the S4 segment in K+ channels is located in the inner (outer) portion of the membrane. These two general configurations can be thought of in terms of ranges for the parameter bK, namely, bK < 1/2 for inward, and bK > 1/2 for outward rectification (Figure 2).
In general, ion channels are typically formed by different subunits, that may combine in different ways, resulting in structural changes that may restrict the flow of ions through them, causing rectification. For instance, non-NMDA glutamatergic receptors that can be activated by kainic acid and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) conduct Na+, K+, and Ca2+, with different permeabilities depending on the subunits that form the receptor (Hollmann et al., 1991). The reason is that the specific combination of GluR subunits forming the receptor restrict ionic flow in different ways. In particular, the currents recorded in oocytes injected with combinations of GluR1 and GluR3 cRNA have different steady state amplitudes and show different levels of rectification (Figure 3).
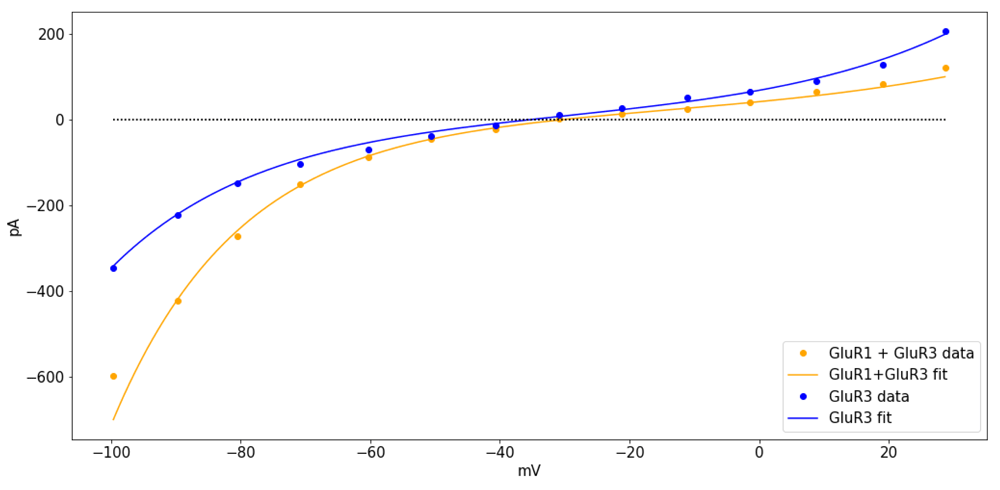
Currents were recorded from oocytes injected with GluR3 cRNA (blue), or a combination of GluR1 and GluR3 cRNA (orange), in Ca2+ ringer solution after activation by AMPA. The curves were fit with(vo, b, rq) = (−30, 0.45, 21), for GluR3 and (vo, b, rq) = (−35, 0.35, 20), for GluR1+GluR3. The data was digitized from Figure 3B in the article by Hollmann et al. (1991), using the ginput function from the python module matplotlib (Hunter, 2007) see Supplementary File 2.
Primary active transport. The Na-K ATPase is a primary active transporter that uses the energy from the hydrolysis of one molecule of ATP for the uphill transport of Na+ and K+ (De Weer et al., 1988). The kinetics of the Na-K ATPase can be assumed to translocate 3 Na+ ions outward and 2 K+ ions inward (ηNaK = 1) with a reversal potential vNaK = vATP + 3vNa − 2vK (see Table 1) in a single transport event (Chapman, 1973; Gadsby et al., 1985; Garrahan & Glynn, 1967; Post & Jolly, 1957). Importantly, the transport kinetics of the Na-K ATPase and by extension, the current, reverse for potentials smaller than vNaK (De Weer et al., 1988).
The current-voltage relationships recorded from Na-K ATPases in guinea pig ventricular cells are shaped as hyperbolic sines (Gadsby et al., 1985). Those currents would be fit with bNaK ≈1/2, yielding currents of the form
The voltage-dependence of the Na-K ATPase currents is reported to show a plateau as v increases past the reversal potential for the current, in response to steroids like strophandin (Nakao & Gadsby, 1989). In such cases, the Na-K ATPase current can be assumed to be inwardly rectifying and fit with values of bNaK ≈ 0, so that,
or alternatively,
The rectification for the Na-K pump ATPase has also been reported to occur in small neurons of the dorsal root ganglion in rats (Hamada et al., 2003). The alternative expression (25) also explains qualitatively different behaviors of the Na-K current as a function of the transmembrane concentrations of Na+ and K+. For instance, if either [Na]1 or [K]0 increase and v > vNaK, then the amplitude of iNaK would increase at a smaller rate of change in comparison to when v < vNaK, which grows exponentially in size. This is also in line with reports of non significant changes in the transport by Na-K ATPases in response to elevated intracellular Na+ during heart failure (Despa et al., 2002), in which the transmembrane potential is likely to be depolarized.
Secondary active transport. An example of a pump that mediates secondary active transport is the Na-Ca exchanger, which takes 3 Na+ ions from the extracellular compartment in exchange for one intracellular Ca2+ ion in forward mode (Pitts, 1979; Reeves & Hale, 1984). The reversal potential for the current is vNaCa = 2vCa − 3vNa, with ηNaCa = 1. Assuming bNaCa = 1/2, the Na-Ca current is
The driving force v − vNaCa could reverse in sign with large enough increases in the intracellular concentration of Ca2+, or in the membrane potential. As a result, the current could have a dual contribution to the change in transmembrane potential, as predicted by some theoretical models of cardiac pacemaker activity (Rasmusson et al., 1990a; Rasmusson et al., 1990b).
Electrodiffusive transport. Consider transmembrane electrodiffusive transport of a single ionic type x, with zx and vx representing the valence and the Nernst potential for x-ions, respectively. In this case, the reversal potential satisfies
which means that ηx can be factorized in the argument for the exponential functions and the general expression (16) can be rewritten as
In the absence of rectification (bx = 0.5) the formula simplifies to
since hyperbolic sines change signs if their arguments do. For calcium channels,
See Pickard (1969); Jacquez & Schultz (1974) Table 1 for other examples.
The applicability of the general formulations described above is illustrated next with models of cardiac and neuronal membrane potential.
To show the application of the formulations discussed earlier, let us build a general model of transmembrane potential dynamics with currents generated by M different electrogenic transport mechanisms. For simplification purposes, consider only one such mechanism, labeled as l, with plNl active sites, where Nl is the number of membrane sites where the lth transport mechanism is found, and pl is the proportion of active sites (might be voltage or ligand dependent). Then the total current mediated by the lth mechanism in a patch of membrane can be written as āl pl φl (v) with a¯l = qNl rl (in pA/µm2), and
where vl/ηl is the reversal potential for the lth current, l ∈ {1, ..., M}. There is experimental evidence for some ion channels that supports the replacement of āl as a constant (Nonner & Eisenberg, 1998). The time-dependent change in transmembrane potential can written as
with v in mV and al = āl/CM in mV/mS (pA/pF) represents the current amplitude for the lth transport mechanism, normalized by the membrane capacitance, for l ∈ (1, ..., N). Only electrogenic transport mechanisms are included.
The pacemaking dynamics of cells in the rabbit sinoanatrial node (Figure 4) can be modeled using low dimensional dynamical systems based on the assumption that v changes as a function of a combination of channel-mediated electrodiffusion and pumping mechanisms involving Ca2+, K+, and Na+ (Herrera-Valdez & Lega, 2011; Herrera-Valdez, 2014). Explicitly, Ca2+ transport is mediated by L-type Cav13 channels (Mangoni et al., 2003) and Na+-Ca2+ exchangers (Sanders et al., 2006). K+ transport is mediated by delayed-rectifier voltage-activated channels (Shibasaki, 1987), and Na+-K+ ATPases (Herrera-Valdez & Lega, 2011; Herrera-Valdez, 2014).
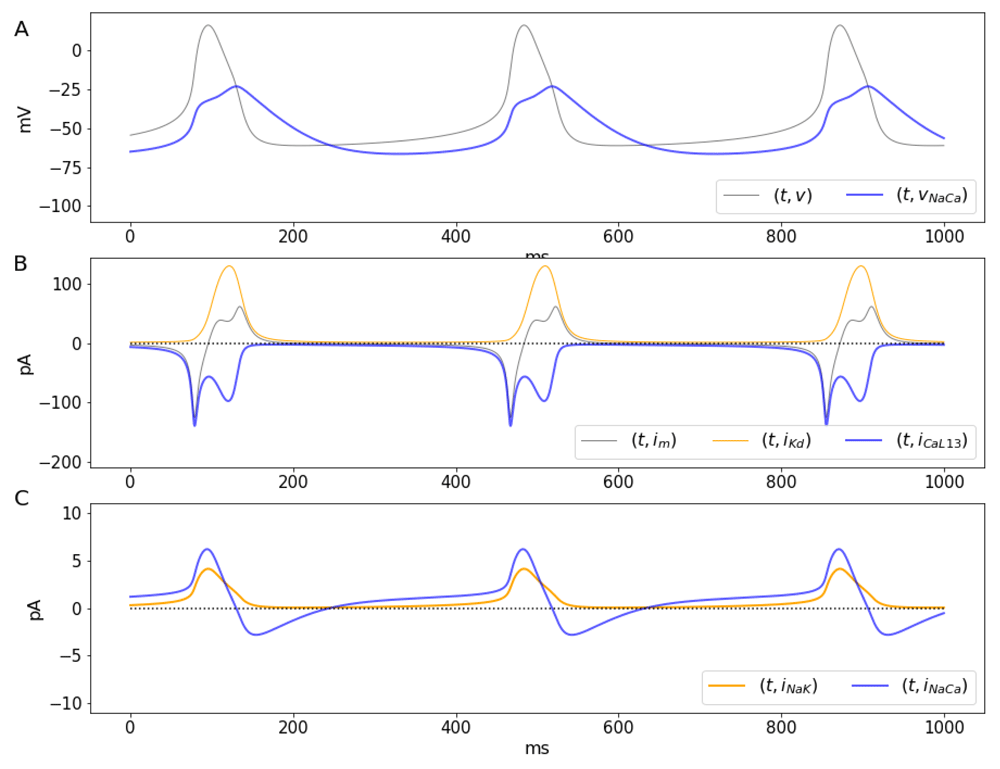
A. Transmembrane potential (gray) and the reversal potential vNaCa (blue) as a function of time. B. Dynamics of large calcium (blue) and potassium (orange) currents in pA. The total current shown by a gray line labeled as im. C. Currents mediated by NaK ATPases (orange) and Na-Ca exchangers (blue), respectively. Notice the carrier mediated currents (C) are about one order of magnitude smaller than those mediated by channels shown (B).
The temporal evolution for v is then described by
where w represents the proportion of activated K+ channels and also, the proportion of inactivated Ca2+ channels (Av-Ron et al., 1991; Herrera-Valdez & Lega, 2011). The variable c represents the intracellular Ca2+ concentration. The transmembrane currents, normalized by the membrane capacitance, given by
and φx is a difference of exponential functions as defined above, with x ∈ {NaK, NaCa, KD, CaL}. It should be noted that these currents are normalized by the membrane capacitance, so ix was replaced by Jx for x ∈ {NaK, NaCa, KD, CaL} to avoid confusion. In this model, the activation for the L-type Ca2+ channels is assumed to be fast and described by a voltage-dependent steady state Fm (Herrera-Valdez & Lega, 2011). The activation of K+ currents recorded in voltage-clamp experiments often displays sigmoidal time courses that resemble logistic population dynamics (Covarrubias et al., 1991; Hodgkin & Huxley, 1952; Tsunoda & Salkoff, 1995). The proportion of activated K+ channels is therefore represented by the variable w, with dynamics evolving according to
where Fw and Rw represent the voltage-dependent steady state and rate (1/ms) for the opening of Kd channels (Willms et al., 1999). The steady state for the activation of voltage-dependent channels in the model is described by the function
which increases as a function of v displaying a sigmoidal shape, controlled by parameters gu and vu for the steepness and the half-activation potential, respectively, for u ∈ {m, w}. The rate of activation for K+ channels is a voltage-dependent function of the form
where bw represents a bias in the conformational change for activation. The function Rw has the shape of a hyperbolic cosine when bw is 1/2. During pacemaking oscillations, the intracellular Ca2+ concentration does change at least 10-fold (Rasmusson et al., 1990a; Rasmusson et al., 1990b). Therefore, the system includes an equation for the dynamics for c, assuming linear dynamics converging to a steady state c∞ in the absence of Ca2+ fluxes, but increasing proportionally to the total transport of Ca2+ ions through L-type channels and Na+-Ca2+ exchangers (Figure 4).
Explicitly,
where kc (µM/mV) represents the impact of the transmembrane Ca2+ fluxes on the intracellular Ca2+ concentration. No buffering mechanisms are explicitly modeled, but included implicitly in the decreasing term −rc c. The minus sign in front of kc accounts for the fact that the sign of the JCaL is negative. The sign in front of JNaCa is because the forward flux of Ca2+ mediated by the Na-Ca exchanger is opposite to that of electrodiffusive Ca2+. The transmembrane concentrations of Na+ and K+ across the membrane are assumed to change negligibly (Herrera-Valdez & Lega, 2011; Rasmusson et al., 1990a; Rasmusson et al., 1990b).
The solutions of equation (37)–equation (40) with parameters as in Table 2 reproduce important features of the membrane dynamics observed in the rabbit’s central sinoatrial node, including the period (ca. 400 ms), amplitude (ca. 70 mV), and maximum ∂t v (<10 V/s) of the action potentials (Zhang et al., 2000).
The amplitudes al can be thought of as āl/CM where CM is a constant that represents the rate of change in charge around the membrane as a function of v, and l ∈ {CaL, K, NaK, NaCa}.
The solutions of the system show a number of interesting features related to ionic fluxes. First, the Na-Ca current reverses when v = vNaCa (Figure 4A, blue line). During the initial depolarization and until the maximum downstroke rate, approximately, vNaCa < v, which means JNaCa > 0, so that Ca2+ extrusion by the Na-Ca exchanger occurs only for a brief period of time during the downstroke and also after each action potential (Figure 4C, blue line). Second, as previously reported in different studies involving spiking dynamics, the time course of the Ca2+ current shows a partial inactivation with a double peak (Figure 4B, blue line) around a local minimum (Carter & Bean, 2009; Rasmusson et al., 1990a; Rasmusson et al., 1990b), and in agreement with data from voltage-clamp experiments (Mangoni et al., 2006). A number of models have made attempts to reproduce the double activation by making extra assumptions about gating (Rasmusson et al., 1990a; Rasmusson et al., 1990b). For instance, some models include a second activation variable, or the multiple terms in the steady state gating, or in the time constant for activation or inactivation. However, the explanation for the double peak can be much simpler. The calcium current JCaL is a negative-valued, non monotonic function for v < vCa, which can be thought of as a product of a amplitude term that includes gating and the function φCaL. The normalized current JCaL has a local minimum (maximum current amplitude) around -10 mV (Figure 4B, blue line and Figure 5A, blue line), after which the current decreases, reaching a local maximum as the total current passes through zero, at the peak of the action potential around 10 mV, (Figure 4B, where ∂t v = 0). The first peak for the Ca2+ current occurs when v reaches the maximum depolarization rate (Figure 5B). As v increases (e.g. upstroke of the action potential). The second peak for the current occurs as the membrane potential decreases, and passes again through the region where the maximal current occurs (local minimum for JCa). The two local minima for JCaL represent peaks in the Ca2+ current that have different amplitudes due the difference in the time course of v during the upstroke and the downstroke of the action potential (Figure 5A, blue line, and Figure 5B, where ∂t v = 0). It is important to remark that the dual role played by w is not the cause of the double activation. This is illustrated by analyzing the behavior of a non-inactivating JCaL without the inactivation component, (Figure 5A, gray line). The double activation can also be observed in models in which the activation of K+ channels and the inactivation of Ca2+ or Na+ channels are represented by different variables (Rasmusson et al., 1990a) and in dynamic voltage clamp experiments on neurons in which there are transient and persistent sodium channels (Carter & Bean, 2009).
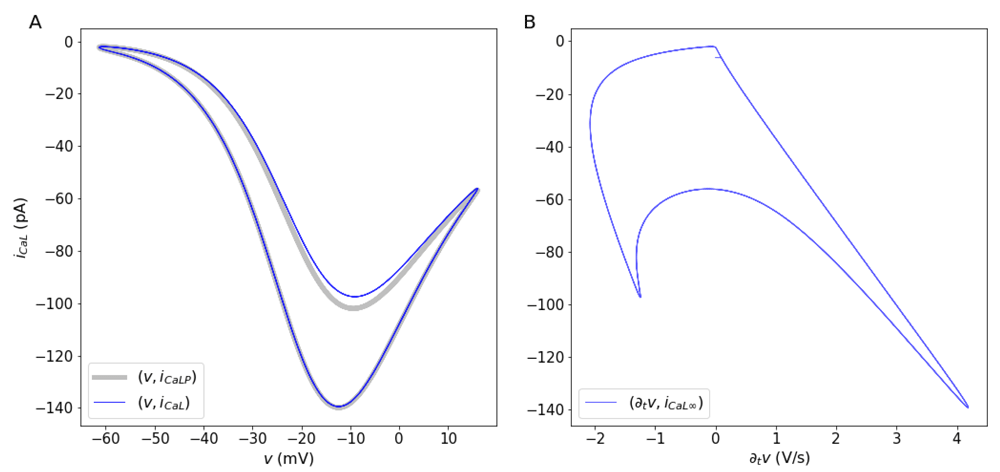
A. Behavior of the inactivating L-type Ca2+ current with respect to the transmembrane potential (blue line) and a non-inactivating current (gray line). Notice that two points with locally maximum current amplitude occur during the action potential. The local minimum with larger current values occurs during the upstroke of the action potential. The local minimum with smaller current values occurs during the downstroke of the action potentials. (B) Calcium currents as a function of the time-dependent change in v. The maximum rate of change for v occurs when the calcium current reaches its maximum amplitude.
The double peak in the Ca2+ current is reflected in the intracellular Ca2+ concentration (Figure 6, gray line), and by extension, on the Nernst potential for Ca2+ (Figure 6, blue line), which display two increasing phases and two decreasing phases, respectively. The first and faster phase in both cases occur during the initial activation of the L-type channels. The second phase occurs during the downstroke, as second peak of the Ca2+ current occurs. As a consequence, the reversal potential for the Na-Ca exchanger, vNaCa = 3vNa − 2vCa (Figure 6, orange line) also has two phases, this time increasing. Increasing the intracellular Ca2+ (Figure 6, gray line) concentration decreases the Nernst potential for Ca2+, and viceversa. By extension, the reversal potential for the Na-Ca exchanger, vNaCa = 3vNa − 2vCa becomes larger when c increases. Ca2+ enters the cell in exchange for Na+ that moves out when v > vNaCa, during most of the increasing phase and the initial depolarization phase of the action potential (blue lines in Figure 4A and C, and Figure 6).
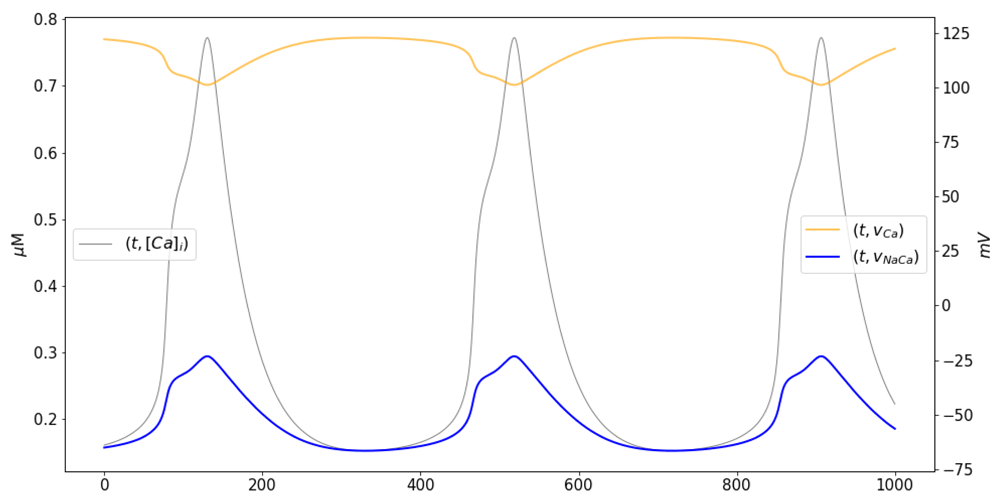
Time courses of the intracellular calcium concentration (gray, left axis), the Nernst potential for Ca2+ (orange, right axis), and the reversal potential for the Na-Ca exchanger (blue, right axis). Notice the two phases of calcium increase that occur in agreement with the double peak observed in the calcium current (see Figure 4B, blue trace).
A general, macroscopic model for transmembrane fluxes has been derived by directly calculating the work required to transport molecules across the membrane. The derivation is based on a general thermodynamic scheme that takes into account the rate, stoichiometry, and the direction in which the molecules are transported across the membrane. These biophysical parameters are then combined to write expressions for directional fluxes based on van’t Hoff (1884) and Arrhenius (1889) formulations, weighted as in the Butler/Erdey-Gruz/Volmer equation (Butler, 1924; Erdey-Grúz & Volmer, 1930). The result is a general description (Equation 16) of the transmembrane molecular flux as a difference of exponential functions, that describes the transport dynamics in the "forward" and "backward" directions, relative to a source compartment. The two exponential functions depend on a common expression involving the transmembrane concentrations of the molecules being transported, and possibly the transmembrane potential when transport is electrogenic.
Rectification, an asymmetry in the flow, is typically modeled modifying the dynamics of the gating variables of the current. The general formulas for transmembrane transport include a bias term b that controls the relative contribution of inward and outward components the transport. Hence, different types of rectification can be described by favoring one of the directions for transport, conceptually in line with the "anomalous rectification" originally reported by Katz (1949) for K+ in muscle cells. The bias term is not part of any gating mechanism. Instead, it represents the asymmetry in bidirectional flux Based on the work of Riedelsberger et al. (2015), the inward (outward, respectively) rectification in K+ channels occurs when the fourth transmembrane segment of the channel (S4) is located closer to the intracellular (extracellular) portion of the membrane in its open configuration. There are other reports that show that asymmetries in bidirectional transport occur as a consequence of changes in the three dimensional structure of the protein mediating the transport (Halliday & Resnick, 1981; Quistgaard et al., 2013). Therefore, the rectification term can be thought of as representing a structural component of the transmembrane protein through which molecules move (Figure 2). Outward rectification in K+ channels can be explained, for instance, by biasing the flux of K+ the forward (outward) direction (bK > 1/2). Instead, inward rectification can be obtained by biasing the transport in the backward (inward) direction (bK < 1/2). It is important to remark that non-rectifying currents with b = 1/2 are nonlinear functions of ∆G, which shows that the nonlinearity of the current-voltage relationships is not the defining characteristic of rectification; as argued in some textbooks (see Kew & Davies, 2010).
The formulation for transmembrane flux may be rewritten in different alternative forms that can be found throughout the literature (see Equation (11) and Equation (14), Goldman, 1943; Johnston et al., 1995). Of particular interest, the widely used conductance-based models for current from the seminal work of Hodgkin & Huxley (1952) turn out to be linear approximations of the general current described here (Herrera-Valdez, 2012; Herrera-Valdez, 2014). This explains why the Hodgkin & Huxley (1952) model captures many of the defining features of action potential generation, in spite of modeling ionic currents as resistive. Another interesting case is that electrodiffusive transmembrane currents derived from the Nernst-Planck equation (Nernst, 1888; Planck, 1890), turn out particular cases of the general formulation presented here (see also Herrera-Valdez, 2014, for details). Examples include the constant field approximation (Clay et al., 2008; Hille, 1992; Johnston et al., 1995), the non-rectifying currents proposed by Endresen et al. (2000), and more general electrodiffusive currents that includes a bias term accounting for rectification (Herrera-Valdez, 2014; Johnston et al., 1995).
Possibly of interest to mathematicians working on bifurcation theory, a third order approximation (Equation (17)) resembling the Fitz-Hugh equations (FitzHugh, 1955; FitzHugh, 1961; Fitz-Hugh, 1966), can be used to construct models that give very close approximations to the full model, while keeping biophysical characteristics like rectification and the multiplicative interaction between the slow variable w and the fast variable v. Further, the third order approximation opens the possibility of expanding on the analysis of dynamical systems based on these general formulas to study normal forms and bifurcations. Depending on the ions involved in each transmembrane transport mechanism, the third order approximations for current can be very close to the full function (Figure 2). Since cubic polynomials are less computationally costly than exponential functions, another possible use of the third order approximations could be in the construction of network models that take into account nonlinearities included in the general formulation, but at a reduced computational cost in comparison to the full model. This possibility is currently being tested and will be reported in the near future. A similar comparison has been made between the full model and the conductance-based approximation taking a dynamical systems perspective and also by means of computational simulations in Herrera-Valdez (2012).
One question of interest because of its possible impact on the interpretation of results from existing modeling studies is how does the excitability and the resulting dynamics in a model of membrane dynamics change when using the thermodynamic transmembrane currents or their approximations? The question has been addressed in a study in which two simple neuronal models with currents mediated by Na+ and K+, each equipped with the same biophysical gating properties and the same relative contributions for the currents, but one with currents as in Equation (19), the other with conductance-based currents. The two models display a number of qualitative and quantitative differences worth considering while making the choice of a model in theoretical studies (Herrera-Valdez, 2012). For a start, the two models are not topologically equivalent across many ratios of the relative contributions of K+ and Na+ channels (Herrera-Valdez, 2012); as would be expected by the fact that conductance-based formulations are only linear approximations of the general currents. One of the most notable differences between the general formulation and the conductance-based formula is the contribution of the nonlinear, high order terms from Equation (16), which results in more realistic upstrokes for action potentials and an overall increased excitability; in this case characterized in terms of the minimum sustained current necessary to produce at least one action potential (Herrera-Valdez, 2012). The increased excitability of the membrane with the general formulation is due, in part, to the large, exponential contribution of the open Na+ and Ca2+ channels, but not the K+ channels, to the change in the transmembrane potential near rest. The time course of the Na+ current during the beginning of the action potential with the general model is much sharper than that of the conductance-based formulation, resulting in a faster upstroke of the action potential; and in better agreement with observations in cortex and other tissues (Naundorf et al., 2006). It is important to remark that the sharper increase in the change of the membrane potential shown using the general formulation is a consequence of the nonlinear driving force terms of the current in the general model (the flux term in the general formulation), and not in the activation dynamics for the transient Na+ current. The nonlinearities unraveled by the general formulation could thus be part of the explanation for the observed sharpness at the beginning of the action potential upstroke observed in different experiments. However, such nonlinearities do not rule out other contributions, such as cooperation between Na channels, or the effects of spatial differences in Na-channel densities, as pointed out by Brette (2013) for the case of action potentials in cortical pyramidal cells. The explanation for the sharpness in action potentials may lie in the combination of the nonlinear factors contributed by the flux in the general formulation, channel densities and gating dynamics, and possibly other contributions.
The general formulation for both passive and active transmembrane transport can be thought of as a tool that facilitates the construction and analysis of models of membrane potential dynamics. The generality and versatility of the thermodynamic transmembrane transport formulations is illustrated with a model of the dynamics of cardiac pacemaking (Equation (32)–Equation (39)). Another example with a model for a fast spiking interneuron can be found in Supplementary File 1. The ion fluxes in the model are assumed to be mediated by two different types of voltage-gated channels and two different types of pumps, all represented with the same functional form (see DiFrancesco & Noble (1985); Herrera-Valdez & Lega (2011); Rasmusson et al. (1990b) for examples in which that is not the case).
One important advantage of the general formulation is that it includes the possibility of explicitly estimating the number of channels or pumps mediating each of the transport mechanisms of interest. This has proven to be useful to study the relative contributions of different currents to the excitability of neurons (see Herrera-Valdez et al., 2013) and cardiocytes (Herrera-Valdez, 2014).
Another extension of possible interest is that of modelling the transmembrane transport between organelles and the cytosolic compartment, which can be done by directly replacing the difference cs − ds in Equation (1) with 1 or -1, accounting for the direction of transmembrane motion of molecules relative to the outer compartment. This and other generalizations enable the possibility of studying the interdependence between electrical excitability across tissues and animal species (Herrera-Valdez et al., 2013), and its cross-interactions with metabolism and other processes of physiological importance, all from a general theoretical framework with common formulations.
Implications for experimentalists. One of the main advantages of the general expressions is that fits to ionic currents can be made straight from the voltage-clamp data without much effort, and without having to calculate conductances, which amounts to imposing the assumption that the current to voltage relationship is linear. Fits to experimental currents can then be directly put into equations describing the change in the membrane potential, and model membrane dynamics of interest without having to make many extra adjustments, as it is the case for most conductance-based models restricted to data.
The model for current in Equation (19) has been used to construct simplified models for the membrane dynamics of different cell types using experimental data. Examples include motor neurons in Drosophila melanogaster (Herrera-Valdez et al., 2013), pyramidal cells in the young and ageing hippocampus of rats (McKiernan et al., 2015), medium spiny neurons in the mouse striatum (Suárez et al., 2015), rabbit sinoatrial node cells (Herrera-Valdez, 2014), and other types of excitable cells (McKiernan & Herrera-Valdez, 2012).
A general model that describes physiological transmembrane transport of molecules has been derived by considering basic thermodynamical principles. The model unifies descriptions of transport mediated by channels and pumps, it can model biases in either one of the directions of flow, and it can be easily converted into a model for current in the case of electrogenic transport. As it is desirable in all models, the general expressions can be thought of as extensions of some previous models. In particular, it is shown that the conductance-based model for current turns out to be a first order approximation of the general formulation.
The expressions for current and molecular fluxes across the membrane based on the general formulation can be used to build general models of transmembrane potential using a unified framework (Shou et al., 2015).
All data underlying the results are available, two source data files are included with this article (Supplementary File 2).
This work was supported by UNAM-PAPIIT IA208618.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The author wishes to thank Joceline Lega, Timothy Secomb, and Raphael Gruener at the University of Arizona; Jose Bargas-Diaz and Antonio Laville from the Cellular Physiology Institute at UNAM; and Erin C. McKiernan from the Physics Department at UNAM for all the time spent in discussions that helped to solidify and deepen the ideas presented in this paper.
Supplementary File 1: The Goldman constant field approximation from the general formulation.
Supplementary File 2: CaPermeabilityKA-AMPA-GatedGluR_Hollman et al (1991). Figure 3B GluR3 and GluR1+3.
Supplementary File 3: JuPyTeR notebook with code generating the figures and numerical integration.
1 The transmembrane potential for which there is a zero net flux of s-ions across the membrane, as given by the Nernst-Planck equation, is
2 The change in Gibbs free energy in "standard" conditions of ATP, ADP and Pi at concentrations of 1M and a water concentration of approximately 55M, yields between -28 and -34 kJ/mol, (Bergman et al., 2010)). The second term in Equation (6) depends on a reaction quotient [ADP] [Pi ] /[ATP], which explains deviations of ∆G from the standard change.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Brette R: Sharpness of spike initiation in neurons explained by compartmentalization.PLoS Comput Biol. 2013; 9 (12): e1003338 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 19 May 21 |
||
|
Version 2 (revision) 21 Nov 18 |
read | |
|
Version 1 14 Sep 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)