Keywords
Key words: E-cadherin; adherens junctions; zebrafish epidermis; enveloping layer (EVL), epidermis basal layer (EBL), deconvolution, 3D segmentation
Key words: E-cadherin; adherens junctions; zebrafish epidermis; enveloping layer (EVL), epidermis basal layer (EBL), deconvolution, 3D segmentation
In this version we provided additional data for hexagonal cells of the EVL and analyzed whether the observed differences in the number of hexagonal cells/µm2 between stages were significant.
We carried out the same analysis applied for the global cell packing analysis (Fig 4b in version 2) but only for hexagonal cells of the EVL (Fig 4a in this version). This revealed a significant increase in the average number of hexagonal cells/ µm2 in the EVL markedly between 24 hpf and 31 hpf, supporting that ordering towards hexagonal cell packing geometry stablishes early during embryonic epidermis morphogenesis. Figure 4 has an additional panel and thus the originals were labeled differently and therefore, we provide a new Figure 4 and new Dataset 3 with excel files labeled accordingly.
We also provide a new Figure 3 with asterisks in panel b, to denote the statistical significance of the differences.
See the authors' detailed response to the review by Tony J. C. Harris
See the authors' detailed response to the review by Marc Muller
See the authors' detailed response to the review by James A. Marrs
The skin is the largest organ of the body in direct contact with the environment. It has a complex structure, being constituted by many different tissues. Skin performs functions that are vital in maintaining body homeostasis, such as the control of body temperature and protection from physical damage and bacterial invasion. Sensory axons innervate the skin at early developmental stages enabling the embryo to sense mechanical, thermal, and chemical stimuli1.
In particular, the topology –connectivity, continuity and neighborhood–, and individual cell phenotypes of epidermis define the strength and permeability of the protective barrier between the organism and its environment. Although epidermis structure varies between aquatic and terrestrial organisms, its stratification is a common mechanism during development2.
In zebrafish, an immature epidermis establishes soon after the end of gastrulation, constituted by the surface layer, enveloping layer (EVL), and the inner epidermal basal layer (EBL)3,4. EVL arises at mid-blastula stage (2.5 hours post fertilization; hpf) covering the whole embryo5. During epiboly EVL maintains tight joins to the yolk syncytial layer (YSL), and becomes the migration substrate for the underlying deep cells spreading during gastrulation6. At the tail bud stage (8–10 hpf), the epiblast forms the outer germ layer, the ectoderm, characterized as a pseudo-epithelial germ layer. From the non-neural ectoderm arises the EBL, which covers the whole embryonic surface underneath the EVL to form a two-layered epithelium by 10 hpf. At 24 hpf, the epidermis is a distinctive bilayer in which the basal layer actively produces collagen to form the basal membrane and the primary dermal stroma7,8. It is after three weeks that the epidermis becomes further stratified and develops into the adult teleost four-layered epidermal structure: cuticle, surface, intermediate and basal stratums9. In adult zebrafish, the EVL cells are replaced by those derived from basal keratinocytes8. Thus, the epidermis of adult zebrafish, as in mice, derives from basal stem cells, further expanding the similarities of epidermal ontogeny across vertebrates10.
E-cadh is member of a superfamily of cadherins, calcium-dependent cell-cell adhesion molecules forming junctions along the apicolateral membranes of adjacent cells11–13. E-cadh plays a key role in determining cell polarity and differentiation, and thereby in the establishment and maintenance of metazoan tissue homeostasis2,14. As epithelia are constituted by cell phenotypes with the maximum polarity and whose identity is primarily specified by E-cadh, it is key to know its expression in the epidermis establishment and maintenance15. Furthermore, due to the mechano-transduction activity coupled to the acto-myosin cytoskeleton remodeling, E-cadh is involved in processes such as cell division orientation in planar polarized epithelia15 and collective cell migration16. Relevant as well is that E-cadh has been characterized as a potent suppressor of invasion and metastasis in epithelia17, which are usually located in direct contact with mutagenic and/or carcinogenic agents responsible for 85–90 % of human cancers18.
In zebrafish, E-cadh transcripts and proteins are maternally deposited. Reduced levels of the maternal and zygotic protein have been proved to delay epiboly progression with lethal phenotypes5. E-cadh is required for blastomeres adhesion during the cleavage stage and later during gastrulation and epiboly19–21. Indeed, as epiboly proceeds, EVL directs cell migration and the spreading of cells of the deep cells layer (DCL) in a process that requires dynamic cell contacts remodeling mediated by E-cadh6. Once the bi-layered epidermis is established, E-cadh role becomes more refined by keeping its integrity while actively remodeling cell-cell contacts within each layer. At tissue scale, this leads to cell rearrangements which establish regular geometric patterns, and loss of E-cadh results in altered epidermis topology5,14,22. Strikingly, scarce knowledge exists regarding epidermal spatiotemporal expression of E-cadh after epiboly stages. By combining 3D-deconvolution and segmentation of AJs in epidermal cells we were able to obtain a quantitative profile of E-cadh expression during normal epidermis morphogenesis from embryonic-to-larval life of zebrafish.
Zebrafish strain of T/AB genetic background was used as wild-type. Male and female adults of 8-months-old were obtained from the Institute of Molecular and Cellular Biology of Rosario (IBR-CONICET-UNR), Argentina, and maintained at 28°C on a 14-h light/10-h dark cycle. Adult fishes were kept in rectangular glass tanks of 12 liters at a density of (1–2 fishes/liter). In each tank, chlorine free water was constantly aerated and filtered (ATMAN hang On filter HF 0100), and renovated by 1/3 twice a week, water temperature was maintained with a heater (Atman 200W). Water pH was kept between 7.8–8.2, salinity was maintained between 350–600 TDS and nitrates were controlled using biological films included in the filtering system. Fishes were fed twice a day with dried flakes (TetraMin) and twice a week with freshly hatched artemia cysts.
After breeding, laid eggs were collected and maintained at 28°C. Then, embryos and larvae were staged according to Kimmel et al.23. Around 20 to 25 embryos were collected at 2.5, 18, 24, 31, 48 and 72 hpf, then dechorionated and sedated with buffered tricaine methylsulfonate (MS-222, Sigma) prior to fixation. Approximately, 10–15 fixed embryos per stage were processed for immunofluorescence detection of E-cadh.
Adults and embryos were handled according to the ARRIVE guidelines and to the national guidelines from the Advisory Committee on Ethics of the Facultad de Bioquímica y Ciencias Biológicas de la Universidad Nacional del Litoral, Santa Fe, Argentina (Res. 229 and 388/2006).
All embryos were fixed in toto in Carnoy solution at room temperature (RT) for at least 2 h and processed according to Izaguirre et al.24. Briefly, they were washed in PBS and permeated in 1% Triton X-100/PBS pH 7.4 for 1 h. Then, washed in PBS pH 7.4 and incubated in normal goat serum (catalogue number: S-1000 Vector Laboratories, Burlingame, CA) for 45 min, followed by overnight incubation with primary antibody anti E-cadh at 4°C, three washes in PBS, and incubation with secondary goat anti-mouse IgG-FITC antibody at RT in darkness for 2 h. Finally, they were rinsed in PBS and mounted in 50% Glycerol-PBS for microscopy imaging. Embryos directly incubated with secondary antibody and normal goat serum, were used as negative controls.
Antibodies. The 36/E-cadh monoclonal antibody recognizes the cytoplasmic domain of human E-cadh, regardless of phosphorylation status (clone 36 mouse IgG2a, catalogue number: 610181 Transduction Laboratories). It was diluted 1:150 and revealed with secondary goat anti-mouse IgG-FITC antibody (Sigma, catalogue number: F8771, St. Louis, MO) used at 1:100 dilution.
The spatial distribution of E-cadh in zebrafish epidermis was analyzed by fluorescence microscopy followed by image deconvolution and cell segmentation in 3D. The trunk was selected for the ease of orientation and image acquisition within the studied periods. Images were acquired with an inverted wide field sectioning microscope Olympus IX83 coupled to a digital camera CMOS-ORCA-Flash 2.8 (Hamamatsu), and commanded by Olympus Cell Sens software v. 1.13. Raw images were processed using FIJI v. 3.0. Sampling in xy was 0.182 µm with z-step every 0.33 µm. The epidermis was completely scanned along the trunk region. Lamp power was set at 12 %, and exposure time was experimentally determined and fixed in 370 ms, in order to avoid pixel intensity saturation and to minimize photobleaching.
Deconvolution was applied to restore fluorescence, which improved contrast and z-resolution, enabling better definition of E-cadh in AJs for subsequent application of the 3D-segmentation tool. Quantification of E-cadh fluorescence intensity was carried throughout the epidermis bilayer (~ 6 μm) in calibrated 3D-ROIs set at 2500 µm2 × 0.33 µm × 20 slices (16500 µm3). First, deconvolution was performed on individual 3D-ROI by applying Richardson-Lucy algorithm25 running under the open source Deconvolution Lab 2 v 2.0.0, with a theoretical point spread function26. The Trainable Weka Segmentation Plugin v. 3.1.0, a classification tool based on machine learning in FIJI27 was applied on each deconvolved 3D-ROI so as to create a template that would automatically find the cell boundaries by providing trainable examples of membranes and cytosol (set as background). Each segmented 3D stack was further converted into 8-bit binary 3D-mask and multiplied by the corresponding deconvolved 3D-ROI to obtain the final “Result of Classification”. On each classified image E-cadh fluorescence was quantified as the sum of pixel intensities per 3D-ROI and expressed as raw integrated density (RawIntDen). This measurement was performed on at least six 3D-ROIs per embryo to cover the trunk region, in five embryos per developmental stage. The pipeline for the image processing, theoretical psf and classifier model files are available as Supplementary File 1 as well as an example output.
On each classified image, 3D-ROIs of fixed volume (10 μm2 × 3 μm deep) were selected along cell-cell contacts in EVL cells and fluorescence intensity was expressed as RawIntDen/cell-cell contact. To assess the fluorescence intensity in individual cells of the EVL, 3D-ROIs were manually outlined along cell perimeters to include the full membrane width and thickness and expressed as RawIntDen/cell area.
Cell morphology and cell area were assessed in EVL and EBL cells from previously selected 3D-ROIs. Round, 4-, 5-, 6-, 7- and 8-sided cells were counted using the “polygon selection tool” in the individual layers. Mean area was expressed in the image calibrated units. Cell packing index was scored for the EVL and expressed as mean of number of cells/ ROI area (2500 μm2). Area of penta- and hexagonal cells from EBL and EVL were compared for all stages (Supplementary File 2).
Five animals in the specified stages were obtained from three to five independent experiments. Differences in E-cadh levels between developmental stages were analyzed using a Linear Mixed Model (LMM). The assumptions of the model were checked graphically (linearity, homoscedasticity, normality of residuals and independence). The non-normality of the data was tested using the Shapiro-Wilk test. The variable “stage” was considered as fixed effects (24, 31, 48 and 72 hpf). The random effects of the model are the number of embryos per stage (5) and number of 3D-ROIs per embryo (at least 6). This number of ROIs per embryo was estimated in order to cover >90% of the embryo trunk for the selected stages.
Differences in mean cellular area and percentages for the observed polygon types were analyzed using LMM containing the same fixed and random effects but adding the variable “morphology”. The same statistical analysis was performed on the data set for the analysis of cell density and expressed as packing index (mean of number of cells / ROI area).
The Tukey’s test was used for post-hoc pair-wise comparison when an effect or an interaction was found significant. Significant differences are denoted with *p < 0.05, **p < 0.01. Data were analyzed with RStudio software’s version 1.1.453 and plotted with the BoxPlotR application or InfoStat software version 2018.
E-cadh expression pattern was determined in wild type zebrafish during epidermis development from 2.5 (blastula period) to 72 hpf. E-cadh protein was clearly detected in embryos at the blastula stage on epiblast cells (EVL) (Figure 1a). By 18 hpf, during primary organogenesis both epidermis layers are already established. At this stage, E-cadh was observed in AJs in EVL cells and weakly detected in EBL cells (Figure 1b–c). At later stages E-cadh labeling was observed as well as cytoplasmic dots, presumably in endocytic vesicles (Figure 1d,g).

a) at 2.5 hours post fertilization (hpf) b) at 18 hpf; c) Zoom of selection in b) showing a single puncta adherens in contacting EVL cells (arrow) and weak E-cadh detection in the EBL (arrowhead); d,g) at 48 hpf, showing cytoplasmic dot labeling in EVL cells ; e–h) 24 to 72 hpf embryos, displaying E-cadh distribution in trunk, clearly visible in underlying EBL cells from 24 hpf. Images are contrast enhanced maximum intensity projections of 20 optical slices, z-step: 0.33 µm, scale bar: 50 µm. Objective: UPLFLN 40X 1.3 NA oil.
In embryos at 24 hpf, E-cadh labeling was observed in vertices (puncta adherens) as well as in micro-clusters along lateral cell-cell contacts in the EVL (Figure 1e). At this stage the underlying EBL was barely visible, detected as a faint E-cadh immunolabeling. From 31 hpf onwards, fluorescence in the underlying EBL cells was clearly detected (Figure 1 f–h).
During the transition from embryo to larval stages the growing detection of E-cadh along cell-cell contacts parallels noticeable changes in cell size and morphology in the epidermis bilayer (Figure 1, e–h), which were further analyzed.
E-cadh levels were compared in the epidermis layers between stages 24, 31, 48 and 72 hpf, a period during which intense morphogenetic events lead to hatching and significant physiological changes occur for the resulting larvae epidermis to adapt to the aquatic environment. By implementing a 3D-Segmentation algorithm based on machine learning we were able to generate a mask to extract fluorescence intensity values along cell-cell contacts on 3D-ROIs covering the trunk epidermis bilayer and at each developmental stage (Figure 2a–d). With this approach we measured a significant increase of E-cadh levels between 31 and 48 hpf, consistent with the visible detection of E-cadh in the EBL and the visible increase in cell density in the EVL, which was subsequently quantitated.
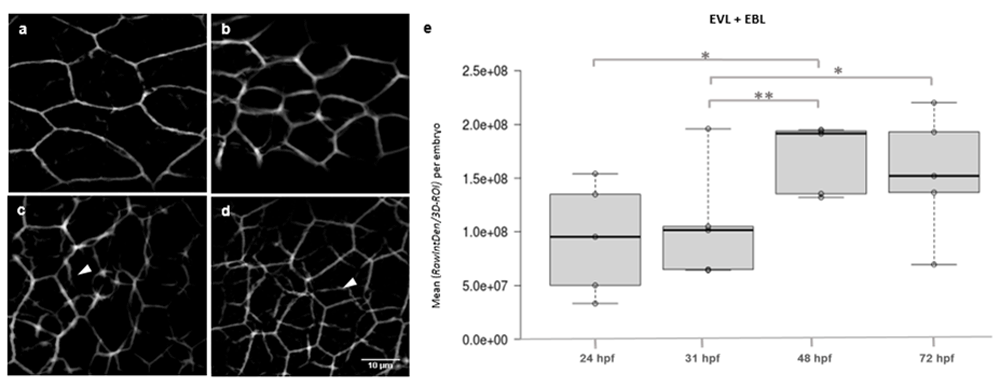
a) 24 hpf and b) 31 hpf, with incipient E-cadh labeling of the underlying epidermis basal layer (EBL) cells; c) 48 hpf; d) 72 hpf, with stronger detection of E-cadh in the EBL (arrow heads). Panels are representative 3D-ROIs classified images shown as sum of intensities in projections of 20-optical section stacks covering the epidermis thickness (6.6 μm); e) Box-plot of means of RawIntDen/ROI per embryo. Objective, 40X, NA 1.3 oil. Statistical significances, ** p<0.01, * p<0.05.
Changes in cell shape, area and density were quantitated in an attempt to correlate the observed increments in E-cadh expression with cell morphology changes characteristics of developing epithelia28. Similar to the other epithelia, the morphogenetic processes leading to epidermis topology development involve cell morphology changes with a predominance of hexagonal geometry in the outermost layer16,24,28. Therefore, the distribution of the cell polygons classes was analyzed in the EVL layer. While round-cells, 4- and 8-side cell polygons were only detected in 24, 31 and 48 hpf stages and represented no more than 7, 15 and 2 % respectively, pentagonal and hexagonal shaped cells predominated in all stages (Figure 3a). Hexagonal cells constituted approximately 34 % of EVL total cells in stage 24 hpf and 45–50% at 31, 48 and 72 hpf with a two-fold increase above pentagonal cells from 31 hpf. Hexagonal cells represented a ~ 50 % of the total cells analyzed consistent with previous reports for other species14.
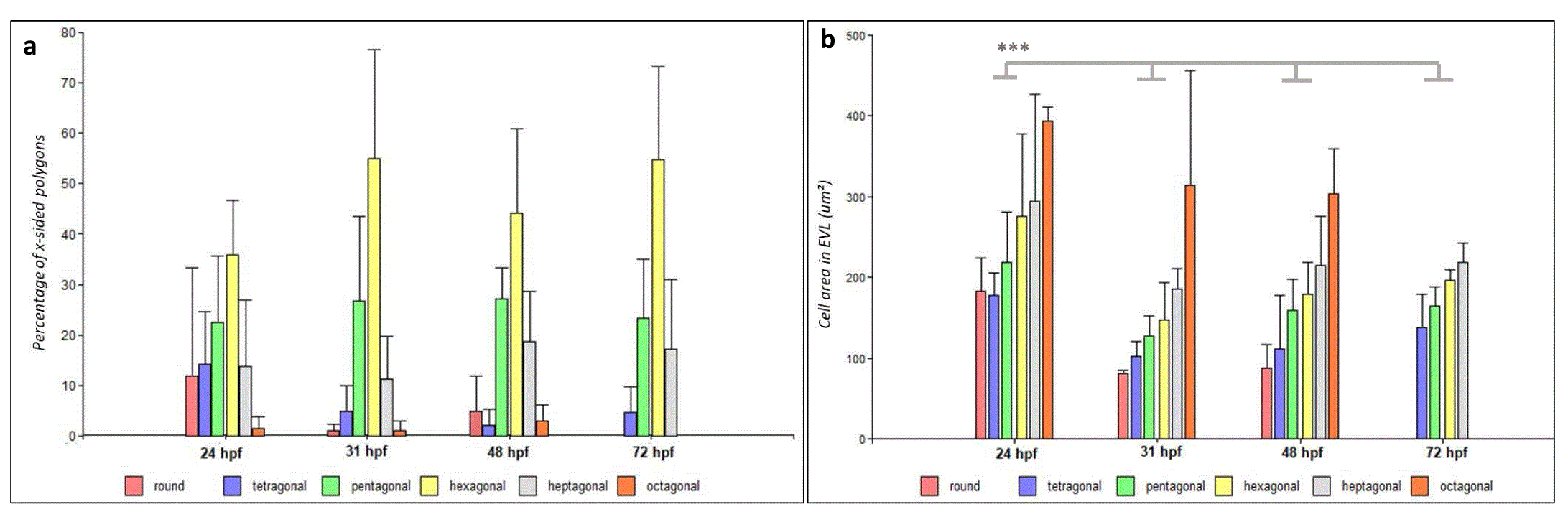
a) Bar graph displaying mean percentages of x-sided polygons of embryos at 24 hpf (n = 81 cells), 31 hpf (n = 147 cells), 48 hpf (n = 133 cells) and 72 hpf (n = 102 cells). Hexagonal and pentagonal cells are the main cell morphologies observed in the EVL. b) Mean area of cell types on EVL at 24 hpf (EVL n= 73), 31 hpf (EVL n= 147), 48 hpf (EVL n= 133) and 72 hpf (EVL n= 102). Significant differences were found in the mean cell area for penta and hexagonal cells between stages (***p < 0.001). Data obtained from 30 3D-ROIs in five animals for each stage. The bars represent mean values ± standard deviations (SD).
For all polygon types assessed in the EVL the mean cell areas decreased within the same polygon type and significantly for pentagonal and hexagonal cells from 24 to 72 hpf (Figure 3b). In the EBL, and despite fewer cells were accessible for area measurements, this tendency was not evident (Supplementary Figure 2). Therefore, the visible increment in cell density in the EVL was characterized by an establishment of hexagonal cell morphology.
We hypothesized then, that the significant increment of E-cadh levels in the epidermis bilayer between 31 and 48 hpf could reflect a contribution of the appearance of more cell-cell contacts per area, the addition of more protein to cell-cell contacts and the emergent detection of the protein in the underlying EBL.
Then, to elucidate whether the increase could be due to the appearance of more cell-cell contacts per EVL area, cell density was estimated globally and for hexagonal cells, and expressed as cell packing indexes (Figure 4a, b). Together, these results showed a significant increment in cell density in the EVL that was characterized by the establishment of hexagonal cell morphology from 24 to 72 hpf. However, there was no significant change in cell density between 31 and 48 hpf, parallel to the increment in E-cadh.

a) Plot of means of cell packing index calculated as the average number of cells/µm2; b) Plot of means of hexagonal cell packing index calculated as the average number of hexagonal cells/µm2); c) Box-plot of means of RawIntDen/cell-cell contact volume; d) Box-plot of means of RawIntDen/average cell area. Statistical significances, ** p<0.01, ***p<0.001.
The separation of the EVL as a sub-stack in individual 3DROIs is not completely free from the fluorescence contribution of the underlying EBL layer. Therefore, to evaluate the contribution of individual EVL cells to the observed differences in global fluorescence intensity, this was quantified in individual cell-cell contacts of fixed volume (3D-ROIs, 10 μm2 × 3 μm deep) in the EVL or in individual cells. With this approach overlapping contacts with the underlying EBL cells were excluded from the measurements. Mean RawIntDen values along cell-cell contacts revealed that more E-cadh may populate individual contacts during embryonic epidermis morphogenesis and contribute to the total fluorescence increase, although differences were not significant between developmental stages (Figure 4c). Then, fluorescence intensity was quantified in individual cells of the EVL manually outlined from the original 3D-ROIs, and expressed as RawIntDen/cell area (Figure 4d). As expected, the expression of E-cadh in the EVL followed a similar pattern as the one obtained for the global bilayer analysis. However, differences between 31 and 48 hpf were not significant. Together this data suggested that the main contribution to the significant increase in E-cadh in the bilayer is due to the growing detection of the protein in the underlying EBL observed at 48 hpf.
Epithelial architecture retains essential features such as apical/basal cell polarization, formation of cell–cell junctions, and the constitution of a paracellular diffusion barrier, all of which enable epithelia to serve a great diversity of biological functions29. The organization of mature epithelia into packaging of hexagonal shaped cells is a feature evolutionarily conserved14,30. This appears to be the optimum arrangement concerning the transduction of forces with minimum energy costs. The role of E-cadh in molecular architecture of epithelia has been studied extensively in animal tissues, partially owing to its decisive play in human cancers31. In frog and fish embryos, as in others, cadherins are the main adhesion factors responsible for regulating the shape of the embryo and its role has been thoroughly described during epiboly and gastrulation32–34. On established animal epidermis, E-cadh performs fine-tuned cell-cell contact remodeling to maintain tissue integrity while the body axis elongates, this is characterized by modulation of cell shape, size and density to achieve the stable hexagonal arrangement28,35,36.
In zebrafish, an immature epidermis is established at 24 hpf, formed by the enveloping layer (EVL) and the epidermal basal layer (EBL). Despite numerous descriptions about E-cadh role in epiboly and gastrulation, there is scarce information about E-cadh distribution in the epidermis beyond this stage and during the embryo to larval transition. During this period body axis elongates from 1 mm at 24 hpf to 3.5 mm at 72 hpf and embryos undergo hatching asynchronously between 48 and 60 hpf23. Once in direct contact with water, the embryonic epidermis is the main protective barrier against pathogens. Therefore, we find it relevant to study the spatiotemporal distribution of E-cadh in zebrafish and elucidate a relationship between E-cadh levels, cell morphology and cell density in the epidermis bilayer from “embryos” to “larvae”.
We implemented a trainable 3D-segmentation tool in FIJI37 to extract fluorescence intensity values from epithelial cells in in toto immunolabeled epidermis. Global expression was estimated from these 3D-segmentation volumes excluding cytoplasm (E-cadh in endosomal or in reticulo-endoplasmic compartments) in the bi-layered epidermis from embryo to larval stages. At present, only one pipeline method was reported for segmentation and tracking of epithelial cells based on the detection of the AJs in voxels in the Drosophila notum and leg epithelium38.
At the membrane level, E-cadh protein concentrates in clusters detected as puncta adherens in cell vertices or as lateral micro-clusters at 24 hpf, that turns into a continuous belt structure from stage 31 hpf onwards, when intracellular E-cadh is also frequently observed in the cytoplasm of EVL cells.
Intensity based analysis showed that growing levels of E-cadh along cell-cell contacts during zebrafish epidermis development correlate with cell morphology changes towards hexagonal geometry. Specifically, within a short period between 24 and 31 hpf, a ~two-fold increase in cell density parallels the appearance of penta- and hexagonal cells together representing ~75 % of the polygons classes similarly to other animal models14.
Global bilayer analysis of E-cadh fluorescence intensity revealed a significant increase in protein expression between 31 and 48 hpf. In an attempt to establish the contribution of individual layers to the observed difference, the outermost EVL layer was analyzed by selecting individual cells or cell-cell contacts without the interference of the fluorescence coming from the basal layer.
Mean E-cadh levels measured in fixed cell-cell contact volumes of EVL cells showed a steady increase from 24 to 72 hpf, but without significant differences between stages. When E-cadh levels were estimated per average cell area in the EVL, a visible increase was observed between 31 and 48 hpf, although non-significant, indicating that the emergent detection of E cadh in the EBL from 31 hpf onwards may indeed contribute to the significant increase in the protein levels measured in the epidermis bilayer.
We cannot overlook that during this embryonic period characterized body elongation, either cell proliferation or adherens junctions remodeling, through active E-cadh trafficking could account for the observed increase in hexagonal cell density in the epidermis39,40. These mechanisms has been well described for the hexagonal cell packing in the developing D. melanogaster pupal wing under polarized trafficking of E-cadh28 and should be further analyzed in vivo within this period in zebrafish.
As a novel outcome, a recent report proposed that tension generated by the E-cadh/AmotL2/actin filaments complexes plays a crucial role in developmental processes such as epithelial geometrical packing as well as generation of forces required for blastocyst hatching both in mouse and human35. In zebrafish, the hatching process is the result of combined enzymatic digestion of the chorion41 together with mechanical forces that drive the embryo out of the yolk sac in a similar way as observed for mouse and human blastocyst hatching. In the present work, a peak in E-cadh membrane level together with an increase in hexagonal cells density in the enveloping layer were detected in the epidermis around the time of spontaneous hatching. Therefore, it is conceivable, that cell morphology remodeling leading to hexagonally packed geometry followed by a significant increase in E cadh may achieve high cell surface compactness and stiffness of the epidermis required for efficient mechanical disruption of the chorion.
The presented results show that during the establishment of embryonic epidermis in zebrafish, growing level of E-cadh protein correlates with increased density of hexagonal cells in the EVL and the detection of adherens junctions in cell-cell contacts in the EBL, significantly between 31 and 48 hpf. This differentiated and compact epidermal tissue is most likely to support mechanical stress prior to hatching which starts around 48 hpf, when the embryo contacts for the first time directly the aquatic environment.
The combination of classical immunofluorescence, image deconvolution with intensity based segmentation in 3D offers a powerful tool to study the spatial arrangement of cell-cell adhesion proteins and cell morphology in bi-layered epithelia that can be applied to cadherin morphants, to other species or processes such as wound healing and re-epithelialization of the skin.
Dataset 1: Raw images for Figure 1 for 2.5 and 18 hours post fertilization (hpf). These can be viewed using FIJI or ImageJ 10.5256/f1000research.15932.d21781942
Dataset 2: Raw and processed images of 3D-ROIs for assessing RawIntDen, cell areas and cell morphology data for 24, 31,48 and 72 hours post fertilization (hpf) for Figure 2a and Dataset 3. Raw can be viewed using FIJI or ImageJ 10.5256/f1000research.15932.d21782043
Dataset 3: RawIntDen, cell areas and cell morphology counts for Figure 2–Figure 4 https://doi.org/10.5256/f1000research.15932.d23650544
This work was supported by the National University of Entre Ríos, Argentina [PID-UNER 6145].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Juan Ignacio Etchart for technical microscopy assistance; Sebastián Graziatti, from IBR-CONICET-UNR, for valuable advice in zebrafish husbandry; and Luciana Erbes and Angel Zeitoune for valuable advice in image processing. We thank Dr. José Biurrum Manresa for critical discussion and assistance with statistical methods, and Prof. Dr. Victor Hugo Casco, for his great support to this research by providing all the necessary equipment and access to the microscopy facilities in LAMAE.
Supplementary File 1: Image processing pipeline for E cadherin fluorescence intensity segmentation and quantitation in 3D-ROIs selected in the trunk epidermis
Click here to access the data.
Supplementary File 2: theoretical psf stack for image processing pipeline
Click here to access the data.
Supplementary Figure 2: Mean cell area of the main cellular types of the enveloping layer (EVL) and epidermal basal layer (EBL) of Danio rerio from 24 to 72 hours post fertilization (hpf) (EVL n=48; EBL n=21), 31 hpf (EVL n=108; EBL n=30), 48 hpf (EVL n=101; EBL n=47) and 72 hpf (EVL n=79; EBL n=45)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Zebrafish development, organogenesis and toxicology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Zebrafish cadherin adhesion
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Zebrafish development, organogenesis and toxicology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 18 Feb 19 |
read | read | |
|
Version 2 (revision) 13 Dec 18 |
read | read | |
|
Version 1 18 Sep 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)