Keywords
Leishmaniasis, Leishmania, Combined, Plumbago capensis, Solanum nigrum
This article is included in the Neglected Tropical Diseases collection.
Leishmaniasis, Leishmania, Combined, Plumbago capensis, Solanum nigrum
Leishmaniases are diseases caused by Leishmania parasites transmitted by the bite of female Phlebotominae sand flies (Piscopo & Mallia, 2006). Desjeux, (2004) indicates that 350 million people are at risk globally, 12 million people are infected with Leishmania parasites and that as many as 2 million new cases occur each year in over 80 Countries.
Studies conducted by Abreu Miranda et al. (2013); de Carvalho & Ferreira (2001) indicates that screening of plant extracts and plant derived compounds is an effective therapy for leishmaniases that avoids exposure to potentially toxic drugs. The World Health Organisation (WHO) (2006), reported that the most pressing research needs for Leishmania control are the search for alternative and cheap drugs for oral, parenteral (injections) or topical administration in shorter treatment cycles, and identification of mechanisms to facilitate access to existing control measures, including health sector reform in some developing countries.
After increasing unresponsiveness to most of the monotherapeutic regimens, combination therapy has found new scope in the treatment of leishmaniasis. The findings of Jha et al. (2005) indicated that the combination of antileishmanial drugs could reduce the potential toxic side effects, prevent drug resistance and increase their efficacy in conjunction. Firooz et al. (2006) and Mishra et al. (2011) reported the superiority of the combination of paromomycin with other drugs for the treatment of visceral leishmaniasis. Studies by Jha et al. (2005) and Firooz et al. (2006) which evaluated combined chemotherapy against visceral leishmaniasis in Kenya using oral allopurinol and endogenous pentostam demonstrated the superiority of the combined drugs. Research shows that natural libraries of plant compounds with recognized antiparasitic activities can be screened and used in development of antileishmanial compounds. This study investigated the effect of combining crude extracts of Solanum nigrum and Plumbago capensis on Leishmania major parasites in vitro.
The proposal for this research work was submitted to the KEMRI Scientific Steering Committee (SSC), for approval and was given ethical clearance (Number: KEMRI/SSC-2028) on the use of the mice as the animal model by the Ethical Review Committee (ERC). All experimental animals at the end of the experiment were sacrificed by injection of 100 µl sagatal and disposed of according to the regulations of Animal Care and Use (ACUC) through incineration.
The in vitro studies were carried out using a comparative study design. Pentostam (Glaxo Operations (UK) Limited, Barnard Castle, UK) and amphotericin B (AmBisome®; Gilead, Foster City, CA, USA) were used as the standard drugs to compare their efficacy with those of the test extracts. RPMI-1640 and Schneider’s Drosophila media (Thermo Fisher Scientific, Waltham, Massachusetts, USA) were used as the control in in vitro experimental chemotherapeutic studies.
Fresh leaves of Solanum nigrum were collected from Kisii and Bungoma, Kenya (0° 40' 49.7352'' S and 34° 46' 37.4196'' E), where the plant is abundant. Plumbago capensis whose activity has been established (Makwali et al., 2015) was collected from Upper Hill area of Nairobi County, Kenya (1°17'59.0"S, 36°48'58.0"E) The plants were transferred to the Center of Traditional Medicine and Drug Research (CTMDR) at KEMRI (Nairobi, Kenya) and dried at 25°C in the shade until they became brittle and attained a constant weight. The dried plants were separately ground using an electric mill (Christy & Norris Ltd., Chelmford, England, model 8) into powder followed by extraction using water and analytical grade methanol (Sigma, 82762). The methanolic extracts were prepared as described by Mutoro et al. (2018a). Immediately, 100 g of ground plant material was soaked in 500 ml of analytical grade methanol for 72 h at room temperature with gentle shaking. The mixture was filtered using Whatman No.1 filter papers (Sigma, Z240079) and concentrated using a rotary evaporator (Cole-Parmer - Stuart - RE400) to obtain dry methanolic extracts. The extracts were coded as A, B and C for methanolic extracts of S. nigrum (Bungoma), S. nigrum (Kisii) and P. capensis, respectively. The aqueous extracts were also prepared as described by Mutoro et al. (2018a). Briefly, 100 g of the dried ground plant material in 600 ml of distilled water was placed in a water bath at 70°C for 1.5 h. The mixture was filtered using Whatman No.1 filter papers and then the filtrate was frozen, dried and weighed. The extracts were coded as D, E and F for P. capensis, S. nigrum (Kisii) and S. nigrum (Bungoma), respectively. The extracts were then stored at 4°C until required for bioassays.
A total of six 8-week-old male inbred BALB/c mice with weights that ranged between 25 and 29 g were obtained from KEMRI. There were eight BALB/c mice per cage (Orchid scientific, SMP 01) in the animal housing kept at 23–25°C under twelve hours in light and twelve hours in dark and were fed on standard diet in the form of mouse pellets and given tap water ad libitum. The mice were handled in accordance with the regulations set by the Animal Care and Use Committee at KEMRI. The mice were used for extraction of peritoneal macrophages that were used for anti-amastigote assay and quantification of nitric oxide produced by macrophages treated with blends of extracts.
The Leishmania major strain (IDUB/KE/94=NLB-144) which was originally isolated in 1983 from a female Phlebotomus dubosqi collected from Marigat, Baringo County in Kenya were used. The parasites were grown to stationary phase at 25°C in Schneider’s Drosophila medium (Fisher Scientific, 21720024) supplemented with 20% heat-inactivated fetal bovine serum (FBS) (Hyclone® USA, SH30071031H), 100 U/ml penicillin and 500 µg/ml streptomycin (Hendricks & Wright, 1979), and 250 µg/ml 5-fluorocytosine arabinoside (Kimber et al., 1981). The stationary-phase metacyclic stage promastigotes were then harvested by centrifugation at 1500g for 15 min at 4°C (Thermo Fisher Scientific 75004061 mySPIN 6 Mini Centrifuge, 1189M94EA). The metacyclic promastigotes were then used for the in vitro assays.
Stock solutions of the crude plant extracts were made as described by Mutoro et al. (2018a). Briefly, plant extracts were made in Schneider’s Drosophila culture medium (Fisher Scientific, 21720024) for anti-leishmanial assays and filtered through 0.22-µm filter flasks in a laminar flow hood (Biological Safety Cabinet). The stock solutions were then stored at 4°C and retrieved later for both in vitro bioassays.
The MICs were determined as described by Wabwoba et al. (2010). Briefly, the L. major metacyclic promastigotes at concentration of 1×106 promastigotes per ml of the culture medium were treated with individual methanolic and aqueous test extracts whose concentrations were 2000 µg/ml, 1000 µg/ml, 500 µg/ml and 250 µg/ml. Similarly, the promastigotes were treated with combined extracts in fixed ratios of 2000:250, 1000:500, 500:1000 and 250:2000. The L. major promastigotes treated with the single extracts and the blends were stained with 100 µL of trypan blue dye while on a microscope slide and observed under the light microscope (XSZ-107 Series Biological Microscope, Sam-Tech Diagnostics) to check their motility and viability compared to the Schneider’s Drosophila medium as the negative control. The lowest concentration of the individual test plant extracts and the blends in which no live promastigotes were observed was the MIC and active ratio for the individual test extracts and the blends respectively.
The anti-amastigote assay was carried out as described by Mutoro et al. (2018a). The peritoneal macrophages were obtained from 4 clean BALB/c mice. The mice were anaesthetized using 100µl pentobarbitone sodium (Sagatal®; Sigma, P3761). The body surface of the mouse was disinfected with 70% ethanol after which it was torn dorso-ventrally to expose the peritoneum. 10µl of sterile cold phosphate buffered saline (PBS) was injected into the peritoneum. After injection, the peritoneum was gently massaged for 2 minutes to dislodge and release macrophages into the PBS. The peritoneal macrophages were then harvested by withdrawing the PBS. The PBS containing the macrophages was washed through centrifugation at 2,000g for 10 minutes and the pellet obtained was re-suspended in RPMI-1640 culture medium. The macrophages were adsorbed in 24-well plates for 4 hours at 37°C in 5% CO2. Non-adherent cells were washed with cold sterile PBS and the adherent macrophages were incubated overnight in RPMI culture medium. Adherent macrophages were then infected with L. major promastigotes and were further incubated at 37°C in 5% CO2 for 4 hours after which they were washed with sterile PBS to remove the free promastigotes, which were not engulfed by the macrophages. This was followed by incubation of the preparation for 24 hours in RPMI-1640 culture medium. The infected macrophages were then treated with combinations of both aqueous and methanolic extracts at fixed ratios of 500:125, 125:125 and 125:500. Pentostam and liposomal amphotericin B were used as positive control drugs to compare the parasite inhibition with that of blends of plant extracts. The medium and blends of test extracts or drug was replenished daily for 3 days. After 5 days, the macrophages were washed with sterile PBS at 37°C, fixed in methanol and stained with 10% Giemsa (Thermo Scientific™, 9990715). The number of amastigotes were determined by counting microscopically at least 100 infected macrophages in triplicate cultures, and the count was expressed as infection rate (IR) and multiplication index (MI) as described by Berman & Lee, (1984) in the formulas below;
IR (%) = Number of infected macrophages per 100 macrophages.
MI (%) =
Measurement of nitric oxide (NO) production was carried out as described by Gamboa-Leon et al. (2007). BALB/c mice peritoneal macrophages at a concentration of 1×105 cells per culture medium were placed in each well in 96-well microtiter plates and allowed to adhere at 37°C in 5% CO2 humidified atmosphere. 2 hours later, the peritoneal macrophages were incubated further in RPMI -1640 medium with 10% FBS for 48 hours in presence of blends of aqueous and methanolic test extracts and the controls. At least 100 µl of macrophage culture supernatants were collected and frozen until when they were required for NO measurement. NO was measured using the Greiss reaction for nitrites (NO2) as described by Hollzmuller et al., 2002. NO2 is one of the products released when the breakdown of NO occurs in the macrophages. NO in the collected supernatants is therefore estimated by quantifying the NO2 content. A nitrite standard reference curve was prepared by dispensing 50 µl of RPMI-1640 with 10% FBS into wells in rows B-H of the first 3 columns in a 96 well plate. 100µM sodium nitrite solution were added to the remaining 3 wells in row A of a 96-well micro titer plate, and immediately a six serial two fold dilutions (50 µl/well) were performed in triplicate columns 1, 2 and 3 down the plate to generate a curve that corresponded to the concentrations 100, 50, 25, 12.5, 6.25, 3.125 and 1.563 µM.
Secondly, 50 µl of the sample supernatant from the wells with macrophages treated with blends of test extracts were added into the wells in triplicate at fixed ratio of 125:125. Greiss reagent A (Fisher Scientific, G7921) were dispensed to all the experimental samples and into the wells containing sodium nitrite solution. Following an incubation of 5 minutes at room temperature, 50 µl of Greiss reagent B (Fisher Scientific, G7921) in water were dispensed into all the wells and incubated for a further 5 minutes at room temperature before measuring the optical density (OD) of the purple/magenta azo compound at 520 nm using micro titer reader (PerkinElmer). The values for the standard nitrite for each of the blend of extracts were read from the standard curve plotted, by reading the NO concentration that corresponds to the absorbance values of the samples.
The data for infection rates and multiplication indices were saved as percentages and then were analyzed using SPSS 13.0 programme. The results were expressed as mean values ± standard deviation (SD). Statistical analysis were done using one way ANOVA and Tukey’s post hoc test and P values < 0.05 were considered significant.
MICs for single extracts and active ratios for the blends of the extracts were detected by looking at the motility and viability of the parasites in the wells as compared to the Schneider’s Drosophila medium (SIM) as the negative control (Table 1 and Table 2).
The MICs of the individual methanolic extracts, S. nigrum from Bungoma (A), S. nigrum from Kisii (B) and P.capensis (C) against L. major promastigotes were 1mg/ml, 0.5 mg/ml and 0.25 mg/ml respectively. The MICs of the individual aqueous extracts, P.capensis (D), S. nigrum from Kisii (E) and S. nigrum from Bungoma (F) were 0.5 mg/ml, 2 mg/ml and 2 mg/ml respectively. In comparison, the MICs of pentostam and amphotericin B against L. major promastigotes were both 0.03125 mg/ml. The Schneider’s Drosophila medium supported the survival of L. major promastigotes to the maximum (Table 3).
The extracts concentration ranged from 2000 µg/ml to 250 µg/ml, while the concentration of the positive controls ranged from 125 µg/ml to 15.625 µg/ml.
The survival levels of promastigotes after treatment with blends of extracts were determined by comparing with survival in both the positive controls (Pentostam and Amphotericin B) and negative control (Schneider’s Drosophila medium). A blend of S. nigrum (Bungoma) and P. capensis (AC) and S. nigrum (Kisii) and P. capensis (BC) methanolic extracts in a ratio 250:2000 inhibited the L. major promastigotes in vitro 100% while ratios 500:1000 and 1000:500 decreased the promastigotes to minimum survival (25%) levels (+) and moderate (50%) levels (++) respectively. Ratio 2000:250 supported the survival of promastigotes to highly moderate (75%) levels (+++) when compared with the controls. A blend of S. nigrum from Bungoma and Kisii (AB) methanolic extracts was efficacious in inhibiting the parasites growth to minimum levels (+) at ratio 1:8 and there was moderate growth at ratios 1000:500 and 500:1000 (Table 4).
++++ indicates maximum (100%) survival, +++ shows highly moderate (75%) survival, ++ shows moderate (50%) survival, + shows minimum (25%) survival and – indicates absence of detectable and live promastigotes when compared to both the positive and negative controls.
| Code | Blends and Controls | Ratio of the extracts based on mica | |||
|---|---|---|---|---|---|
| 2000:250 | 1000:500 | 500:1000 | 250:2000 | ||
| A:B | S. nigrum (Bungoma): S. nigrum (Kisii) methanolic extracts | ++++ | ++ | ++ | + |
| A:C | S. nigrum (Bungoma): P. capensis methanolic extracts | +++ | ++ | + | - |
| B:C | S. nigrum (Kisii): P. capensis methanolic extracts | +++ | + | + | - |
| D:E | P.capensis: S. nigrum (Kisii) water extracts | - | ++ | +++ | ++++ |
| D:F | P.capensis: S. nigrum (Bungoma) water extracts | + | ++ | ++++ | ++++ |
| E:F | S. nigrum (Kisii): S. nigrum (Bungoma) water extracts | ++ | +++ | +++ | ++++ |
| Controls | Concentrations of standard drugs (µg/ml) | ||||
| 125 | 62.5 | 31.25 | 15.625 | ||
| Pentostam | Pento | - | - | - | ++ |
| Amphotericin B | Amph B | - | - | - | + |
| Schneider’s Insect Medium | SIM | ++++ | ++++ | ++++ | ++++ |
A blend of the aqueous extracts of P. capensis and S. nigrum from Kisii (DE) at ratio of 2000:250 led to complete inhibition of parasites growth while ratios 1000:500, 500:1000 and 250:2000 supported growth of the parasites. A blend of aqueous extracts of P. capensis and S. nigrum from Bungoma (DF) at ratio 2000:250 inhibited the growth of the parasites to minimum levels (+) and a blend of S. nigrum from Kisii and Bungoma (EF) supported growth of the parasites at all ratios (Table 4).
Both pentostam and amphotericin B drugs were able to inhibit the growth of L. major promastigotes in vitro at a concentration of 31.25 µg/ml. The Schneider’s Drosophila medium, on the other hand, supported the maximum (100%) growth survival of L. major promastigotes as indicated by four pluses (++++) when compared to the positive controls, pentostam and amphotericin B (Table 4).
When the methanolic extracts were combined in ratios of 500:125, 125:125 and 125:500, the blend of S. nigrum from Kisii and P. capensis (BC) had the lowest infection rate of 46.7% at a concentration ratio of 125:500. Similarly, the methanolic extracts combinations of S. nigrum from Bungoma and Kisii (AB) and S. nigrum from Kisii and P. capensis (AC) resulted to infection rate (IR) of 61.7% at the same ratio (Table 5).
Combinations of the aqueous extracts of P. capensis and S. nigrum from Kisii (DE), P. capensis and S. nigrum from Bungoma (DF) and S. nigrum from Kisii and Bungoma (EF) at the ratio of 125:500 resulted to infection rates of 70.0%, 78.0% and 78.7% respectively. The efficacy of combined aqueous extracts S. nigrum from Kisii and Bungoma (EF) in the ratio of 125:500, in inhibiting the infectivity and multiplication of L. major amastigotes in BALB/c peritoneal macrophages in vitro was lower than all the other blends of extracts (Figure 1 and Table 5). The blend of methanolic extract of S. nigrum from Kisii and P. capensis (BC) performed the best with the multiplication index (MI) of 50.6% at the ratio of 125:500.
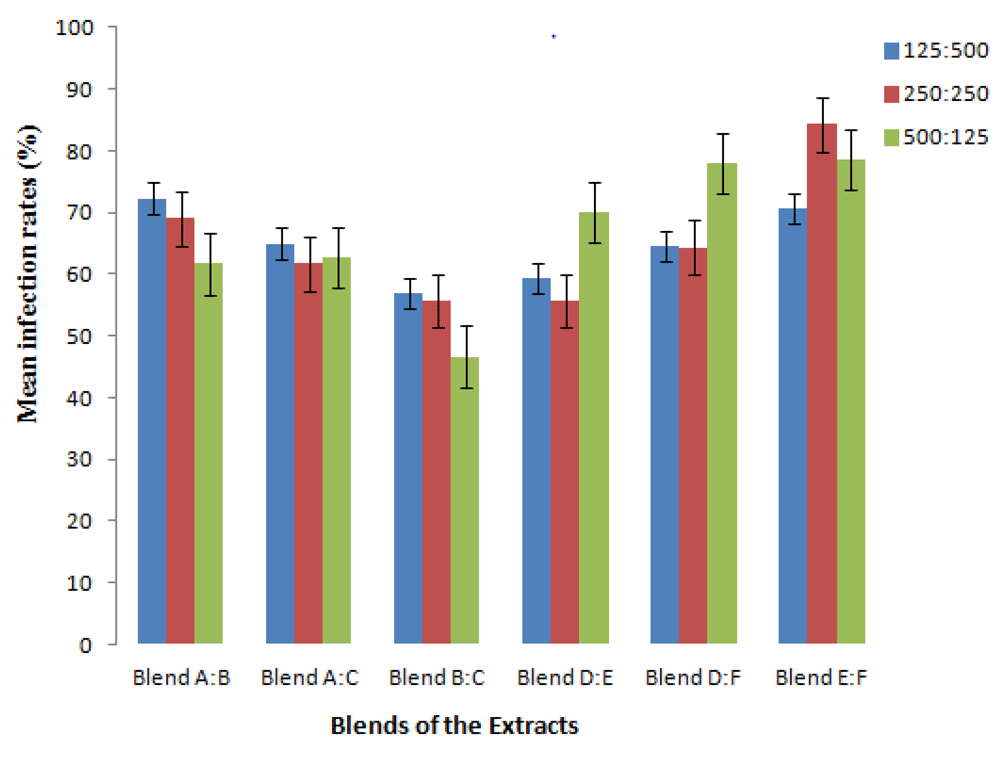
RPMI 1640 medium which had no drug incorporated supported the growth of L. major amastigotes in peritoneal macrophages (Figure 2) more effectively and this was indicated by a high infection rate (IR) of 89.7 % (Table 5). In contrast, the leishmaniasis drugs, pentostam and liposomal amphotericin B inhibited the in vitro survival of L. major amastigotes more effectively and this corresponded to low infection rates of 26.3% and 21.0 % respectively at a concentration of 50 µg/ml (Table 4). The IRs and MIs associated with all the combined extracts were significantly different (P< 0.05) from those of pentostam and amphotericin B at all the concentrations studied.
Nitric oxide (NO) plays a key role as a leishmanicidal effector molecule in host macrophages (Gamboa-Leon et al., 2007). Therefore the effect of the blends of test extracts on NO production was evaluated in vitro. BALB/c mice peritoneal macrophages were incubated in RPMI-1640 medium for 48 hours in the presence or in absence of blends of test extracts. In order to determine the amount of NO triggered by the combined extracts, their optical densities (absorbencies) were determined using Griess Reagent system. All the absorbencies for the combined extracts ranged between 0.034 to 0.041 (Table 6). These OD corresponded to < 5 µM of NO in the standard nitrite curve for the blends of both the methanolic and aqueous extracts. RPMI-1640 medium produced similar negligible levels of NO (Table 7).
| NO (µM) | Standard Nitrite (Positive control) | RPMI (Negative control) |
|---|---|---|
| 0 | 0.021±0.002 | 0.028±0.000 |
| 1.5625 | 0.047±0.001 | |
| 3.125 | 0.076±0.002 | |
| 6.25 | 0.113±0.001 | |
| 12.5 | 0.160±0.003 | |
| 25.0 | 0.266±0.006 | |
| 50.0 | 0.505±0.02 | |
| 100.0 | 0.982±0.003 |
Natural products which have been found to possess antileishmanial activities can prodive alternative treatment for antimonial-resistant Leishmania strains (Monzote, 2009). In cases where the infectious agent fails to respond to single therapy, combined therapy is often adopted. Studies by Melaku et al. (2007); Nyakundi et al. (1994) and Sundar et al., 2008) on leishmaniases reported that antileishmanial drugs combination improved their efficacy and reduced resistance, the dosage required and toxicity levels.
As observed in the current study, the standard drugs were significantly more effective (P≤ 0.05) against Leishmania promastigotes and amastigotes as compared to all the blends of the extracts. It was observed in the present study that all the blends of extracts induced little (< 1.5 µM) production of NO by peritoneal macrophages which might have played a role in the amastigote inhibition.
Freitas-Junior et al. (2012), study demonstrated that miltefosine and amphotericin B or paromomycin combinations were effective against antimony-resistant VL infections. A study conducted by Melaku et al. (2007) indicated that a combination of paromomycin with sodium stibogluconate was effective than when sodium stibogluconate was used alone. Similarly, a study by Ghazanfari and others (2000) demonstrated that garlic extract in combination with glucantime reduced the lesion size caused by Leishmania major more effectively than when they were alone.
Use of herbal drugs as combinations has also been in practice for centuries to treat various infectious diseases. The findings of Yousefi et al. (2009), indicated that a combination of the extracts of Alkana tincturia and Pegunum harmala in a ratio of 1:1 (10:10 µg/ml) showed better in vitro effect against Leishmania major than when single extracts were used. The study conducted by Makwali et al. (2012) indicated that combination therapy with plumbaginaceae extract and triterpenoid saponin extract in combination with acridine and dinitroaniline herbicides resulted in complete clearance of parasitemia from both the lesion site and internal organs of L. major-infected BALB/c mice. Another study by Ndungu et al. (2017) revealed that the water and methanolic extracts of A. secundiflora and P. capensis can be used either separately or in combination as antileishmanial therapeutic agents.
This study has demonstrated that combination therapy using Plumbago capensis in combination with S. nigrum resulted in complete inhibition of the growth of L. major parasites. The emergence of antimonial-resistant Leishmania strains is on the rise and natural products and other plant products that have been tested and found to possess antileishmanial activities may provide alternative treatment. On the basis of these results and considered together with existing studies that have been reported on the safe doses and side effects, combination drug therapy is a promising approach for the treatment of L. major infection.
Dataset 1: Anti-amastigote (macrophage) assays 10.5256/f1000research.15955.d217390 (Mutoro et al. 2018b)
Dataset 2: Quantification of nitric oxide (NO) produced 10.5256/f1000research.15955.d217391 (Mutoro et al. 2018c)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Mechanism of infection & Immunity of Visceral leishmaniasis
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: natural products against leishmaniasis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Sep 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)