Keywords
pregnancy; congenital malformations, hormones
pregnancy; congenital malformations, hormones
We thank the reviewers for their positive comments about our manuscript and we have responded to the points made and revised version 1 of the paper in light of their comments. The major changes affect the introduction which has been revised in line with the peer review comments, We have also made changes to the references due to citation errors; uploaded revised excel data sheets as there was an error in the data in the first version, and made one correction in the text of the results to the ‘Exposure to oral HPTs was associated with a 40% increased risk of all congenital malformations: pooled odds ratio (OR) = 1.40 (95% CI 1.18 to 1.66; P < 0.0001; I2 = 0%).', which incorrectly stated the increased risk as 37%. We have also revised the forest plots (Figures 2-8) as the effect estimates were incorrectly labelled.
See the authors' detailed response to the review by Jesse Olszynko-Gryn and Cyrille Jean
See the authors' detailed response to the review by Olalekan Uthman
See the authors' detailed response to the review by David Healy
Oral hormone pregnancy tests (HPTs), such as Primodos (known as Duogynon in Germany), were available as injections from 1950 and in tablet form in the UK from 1956 onwards, before the modern forms of urine pregnancy tests became available1. Oral HPTs contained ethinylestradiol and large doses of norethisterone (synthetic forms of estrogen and progesterone respectively), the latter in much larger amounts than those included in current combined oral contraceptives (see Table 1). The test principle was that they would induce bleeding similar to menstruation in those who were not pregnant.
| Indication (oral formulation) | Ethinylestradiol dose | Norethisterone acetate dose |
|---|---|---|
| Progestogen-only contraception* | - | 350 micrograms |
| Combined oral contraceptive (Loestrin-20) | 20 micrograms | 1000 micrograms |
| Combined oral contraceptive (Norimin) | 35 micrograms | 1000 micrograms |
| Biphasic combined oral contraceptive (BiNovum) | 35 micrograms | 500/1000 micrograms |
| Triphasic combined oral contraceptive (Synphase) | 35 micrograms | 500/1000/500 micrograms |
| Combined oral contraceptive (Loestrin-30) | 30 micrograms | 1500 micrograms |
| Oral hormone pregnancy test (Primodos) | 20 micrograms | 10 milligrams |
| In endometriosis, dysmenorrhoea, dysfunctional uterine bleeding, and menorrhagia, or to delay menstruation* | - | 10–15 milligrams/day |
| Breast cancer* | - | 40 milligrams/day |
In the UK more than a million women took HPTs2. However, evidence that they should not be used in pregnant women because of a risk of fetal malformations3 led the then Committee on Safety of Medicines in 1975 to conclude that a warning should be added to the Data Sheets, stating that HPTs should not be taken during pregnancy. (Supplementary File 1) Warnings about HPTs in pregnancy first emerged in 1956:4 accumulating concerns over an increased risk of malformations led to their withdrawal in a number of countries at different times. Norway cancelled the indication in pregnancy for HPTs in 1970; when the UK did so in 1978, the manufacturers of Primodos, Schering AG (taken over by Bayer AG in 2008), voluntarily stopped marketing the product; in Germany, Duogynon was taken off the market in 19811.
Since Primodos was withdrawn, the discovery of previously confidential documents has led to renewed concerns about its potential to cause harm5. In 2014, therefore, the Medicines and Healthcare products Regulatory Agency (MHRA) initiated a review, which was published in 2017 and reported that the evidence was insufficient, mixed, and too heterogeneous to support an association between oral HPTs and congenital malformations3.
To date, there has been no systematic review and meta-analysis of oral HPTs, using all the available data, to assess the likelihood of an association. We have therefore performed a systematic review to obtain all relevant data on hormone pregnancy tests and congenital malformations, used meta-analytical tools to obtain summary estimates of the likelihood of an association, and assessed the potential biases in these estimates.
Full details of our search strategy are provided in Supplementary File 2. We searched Medline, Embase, and Web of Science (which yielded German papers and conference abstracts) and searched for regulatory documents online, including the UK Government’s “Report of the Commission on Human Medicines’ Expert Working Group on Hormone Pregnancy Tests”, which includes the original Landesarchiv Berlin Files3, and reference lists of retrieved studies from the start of the databases in 1946 to 20 February 2018.
We used the following search terms without date limits or language restrictions: (Primodos OR Duogynon OR "hormone pregnancy test" OR "sex hormones" OR "hormone administration" OR “norethisterone” OR “ethinylestradiol”) AND pregnancy AND (congenital OR malformations OR anomalies). Several comparable high-dose HPTs were available at the same time as Primodos; we performed additional searches for evidence relating to these (See Supplementary File 3 for List of HPTs included in evidence search).
We included observational studies of women who were or became pregnant during the study and were exposed to oral HPTs within the estimated first three months of pregnancy and compared them with a relevant control group. When a study was described in more than one publication, we chose the publication that contained the most comprehensive data as the primary publication. We excluded studies where the intervention was oral hormones taken for other reasons (e.g., oral contraception) and it was not possible to extract data on hormone pregnancy tests. We did not restrict the language of publication. We checked additional relevant data and extracted them from the secondary publications when necessary.
Two reviewers (CH and ES) applied inclusion and quality assessment criteria, compared results, and resolved discrepancies through discussion with the other authors. We used a review template to extract data on study type, numbers of pregnancies exposed and not exposed to oral HPTs, and types and numbers of outcomes. Where available, we extracted data about the women studied, including ascertainment of cases, age, parity, setting, exposure to other medications, and confounding variables. In case-control studies, if data were reported on more than one control group, we extracted data where possible for non-disease/non-abnormality controls, and combined control groups if necessary.
The primary outcome of interest was all major congenital malformations. We also categorized outcomes for the congenital anomaly in the offspring at any time into congenital cardiac, gastrointestinal, musculoskeletal, nervous system, and urogenital defects, and the VACTERL syndrome (Vertebral defects, Anal atresia, Cardiovascular anomalies, Tracheoesophageal fistula, Esophageal atresia, Renal anomalies, and Limb defects).
We assessed quality using the Newcastle–Ottawa Scale (NOS) for non-randomized studies included in systematic reviews6. The scale assesses the selection of study groups (cases and controls), comparability of study groups, including cases and controls, and ascertainment of the outcome/exposure. Each positive criterion scores 1 point, except comparability, which scores up to 2 points. The maximum NOS score is 9, and we interpreted a score of 1 to 3 points as indicating a high risk of bias7. To determine whether the study had controlled for the most important factors, we selected the items reported in the original paper and resolved disagreements through consensus, using a third author (IO). We examined whether there was a linear relation between methodological quality and study results, by plotting the odds ratios against the NOS scores, using Excel, and assessed the correlations of NOS scores with several confounding variables we collected8.
We calculated study-specific odds ratios for outcomes and associated confidence intervals. We meta-analysed the data using a random-effects model. We assessed heterogeneity across studies using the I2 statistic and publication bias using funnel plots9. We performed a sensitivity analysis by removing single studies to judge the stability of the effect and to explore the effect on heterogeneity10, and we described any sources of variation. We also judged robustness by removing studies of low quality from the analysis. To examine whether the observed heterogeneity could be explained by differences in the NOS score, we also performed meta-regression using the NOS score as the covariate against the log OR as weights for traditional meta-regression using Stata version 14.
We planned subgroup analyses for the timing of administration of HPTs in relation to pregnancy and organogenesis and study design (case-control versus cohort) using Cochran’s Q test. We used RevMan v.5.3 for all analyses, except for meta-regression, for which we used Stata version 14. RevMan and Stata estimate the effects of trials with zero events in one arm by adding a correction factor of 0.5 to each arm (trials with zero events in both arms are omitted). We performed a sensitivity analysis by removing studies with zero events from the analyses.
We followed the reporting guidelines of the Meta-Analysis of Observational Studies in Epidemiology (MOOSE). A completed checklist is available as Supplementary File 411
Members of the Association for Children Damaged by HPTs were involved in the original discussions of this review and provided input to the outcome choices, the search, the location of study articles, and translations. We plan to present the study findings to relevant patient groups and make available lay interpretations.
We retrieved 409 items for screening. After title and abstract screening and removal of duplicates (n = 18), we excluded 354 records as not being relevant to the aim of the review. We assessed the full texts of 37 articles and identified 24 articles for inclusion. Figure 1 shows the PRISMA flow diagram for the inclusion of studies.
The 24 included articles reported on 26 studies (16 case-control studies and ten prospective cohort studies); one article [Nora 78] included two case-control studies and one prospective study. We found no randomized controlled trials. Of these articles, two were unpublished reports (see Supplementary File 5 for full references). The studies included 71,330 women. The case-control studies included 28,761 mothers, 594 of whom were exposed to HPTs; the cohort studies included 42,569 mothers and 3,615 exposures to HPTs. The studies were published between 1972 and 2014, and all were performed either in Europe or the USA. They mostly recruited women and their infants at maternity centres or hospital paediatrics wards.
The choices of controls in the case-control studies varied; they included, at one extreme, healthy infants born on a date close to the case infants and, at the other extreme, infants with malformations other than those under investigation. Among the prospective cohort studies, the populations tended to be women recruited at antenatal clinics or birth centres (See Table 2. Characteristics of included studies).
Of the 26 included studies, three were assigned a NOS score of 3 or below and were therefore judged as being at high risk of bias. One was a case-control study (Laurence 1971, a published abstract as a letter) and two were cohort studies (Fleming 1978 and Haller 1974, both unpublished). The NOS scores ranged from 2 to 9 (median 5). Twelve of the 26 included studies scored 7 to 9 and were judged to be at low risk of bias (see Table 3 of NOS scores in the data files). Item 5 of the NOS score addresses comparability of cases and controls based on design or analysis. Of the 16 case control studies, 12 controlled for the most important factor (item 5a) and nine controlled for important additional factors (item 5b). Of the ten cohort studies, six controlled for the most important factor (item 5a) and four controlled for important additional factors (item 5b). The mean Newcastle–Ottawa scale score was 6.1, indicating an overall moderate risk of bias. Table 2 also shows that seven studies did not report the confounding variables collected (Laurence 1971; Levy 1973; Tummler 2014; Fleming 1978; Haller 1974; Moire 1978; Rousel 1968). NOS scores correlated with the increasing number of confounding variables collected (r = 0.83). Supplementary File 6 shows the funnel plots for all congenital malformations and congenital heart disease; because of inadequate numbers of included studies, we did not use more advanced statistical methods to assess publication bias.
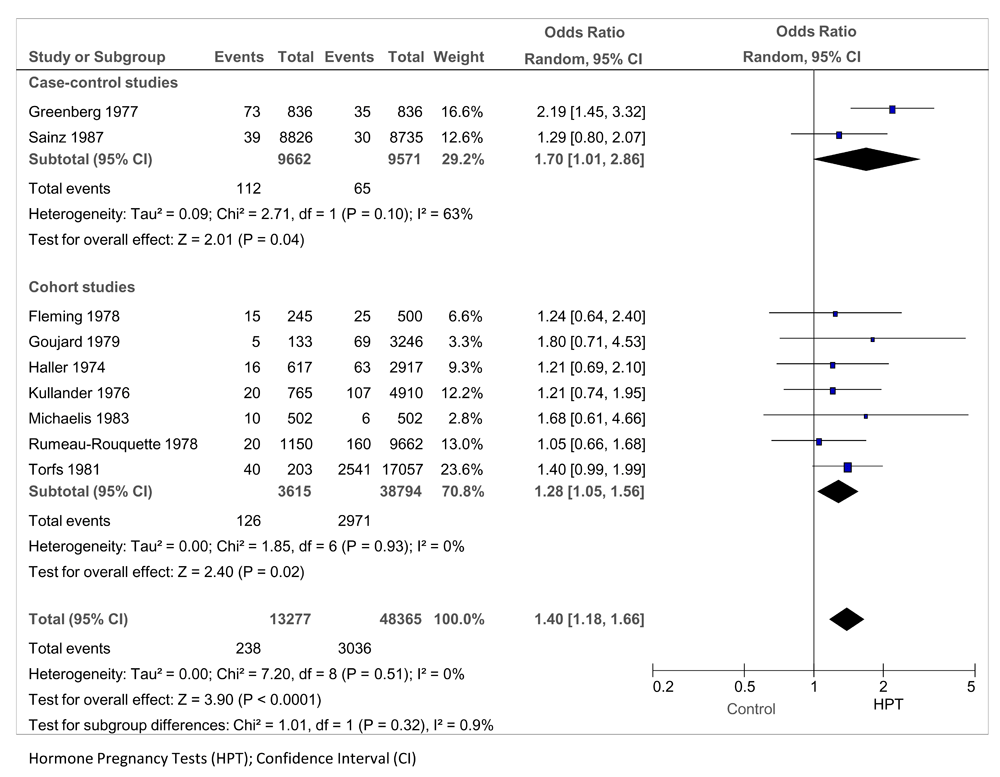
Nine studies, including 61,642 mothers of infants and 3,274 exposed to HPTs, examined the association in pregnancy with all congenital malformations. Two were case-control studies (Greenberg 1977; Sainz 1987) and seven were cohort studies (Fleming 1987; Goujard 1979; Haller 1974; Kullander 1976; Michaelis 1983; Rumeau-Rouquette 1978; Torfs 1981) (Figure 2). Exposure to oral HPTs was associated with a 40% increased risk of all congenital malformations: pooled odds ratio (OR) = 1.40 (95% CI 1.18 to 1.66; P < 0.0001; I2 = 0%). For the two case-control studies only, pooled OR = 1.70 (95% CI 1.01 to 2.86; P = 0.04; I2 = 63%) and for the seven cohort studies, pooled OR = 1.28 (95% CI 1.05 to 1.56; P = 0.02; I2 = 0%). The test for subgroup differences was not significant (P = 0.32). In a post-hoc sensitivity analysis, removing the studies that collected no confounding variables (Haller 74 and Fleming 78, both of low quality) did not affect the significance of the result (OR 1.44; 95% CI 1.18 to 1.75; P = 0.0004, I2 = 11%). The meta-regression showed no association between total NOS score and increased risk (P = 0.51).
Seven studies, including 19,267 mothers of infants and 218 exposed to oral HPTs, analysed congenital heart malformations. Five were case-control studies (Ferencz 1980; Janerich 1977; Levy 1973; Nora 1978-2/3) and two were cohort studies (Hadjigeorgiou 1982; Torfs 1981) (Figure 3). The pooled relative OR = 1.89 (95% CI 1.32 to 2.72; P = 0.0006; I2 = 0%).

In a post-hoc sensitivity analysis, removing one study that collected no confounding variables (Levy 73, a low-quality study) did not affect the significance of the result (OR = 1.88; 95% CI 1.25 to 2.85; P = 0.003, I2 = 12%) For the five case-control studies only, the pooled OR = 1.87 (95% CI 1.23 to 2.85; P = 0.004; I2 = 9%); for the two cohort studies the pooled OR = 1.95 (95% CI 0.44 to 8.69; P = 0.38; I2 = 32%). The meta-regression was not significant (P = 0.94).
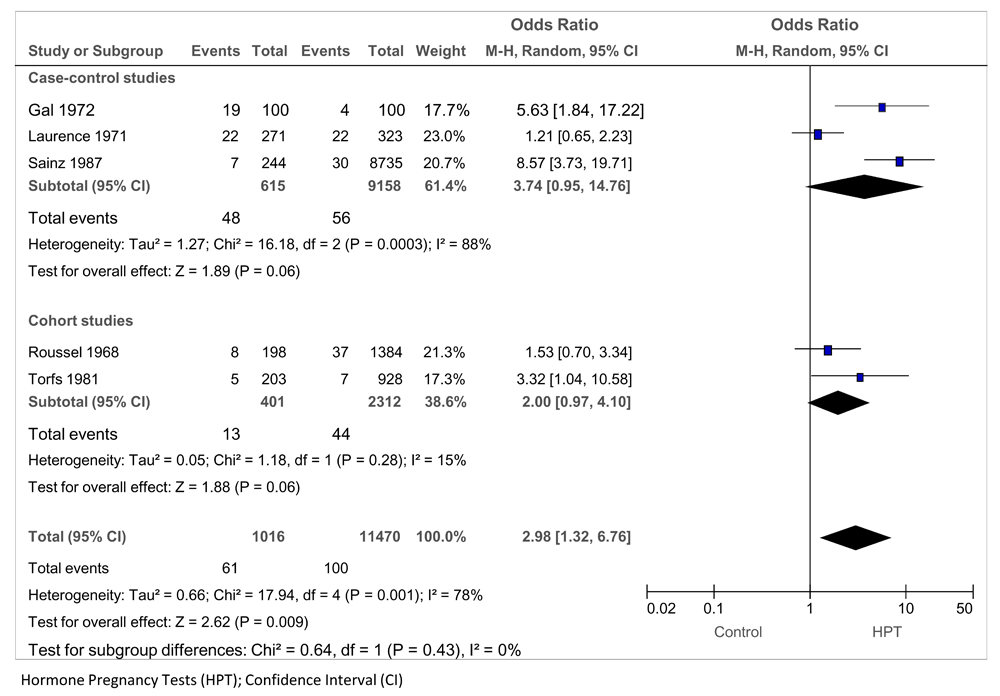
For the association between exposure to oral HPTs and nervous system malformations in the offspring, five studies provided data: three case-control studies (Gal 1972; Laurence 1971; Sainz 1987) and two cohort studies (Roussel 1968; Torfs 1981), including 12 486 mothers of infants and 127 exposed (Figure 4). The pooled OR = 2.98 (95% CI 1.32 to 6.76; P = 0.009; I2 = 78%). In a post-hoc sensitivity analysis, removing the two studies that collected no confounding variables (Laurence 71; Roussel 68) did not affect the significance of the result and removed the heterogeneity (OR 6.04; 95% CI 3.33 to 10.78; P < 0.00001, I2 = 0%).
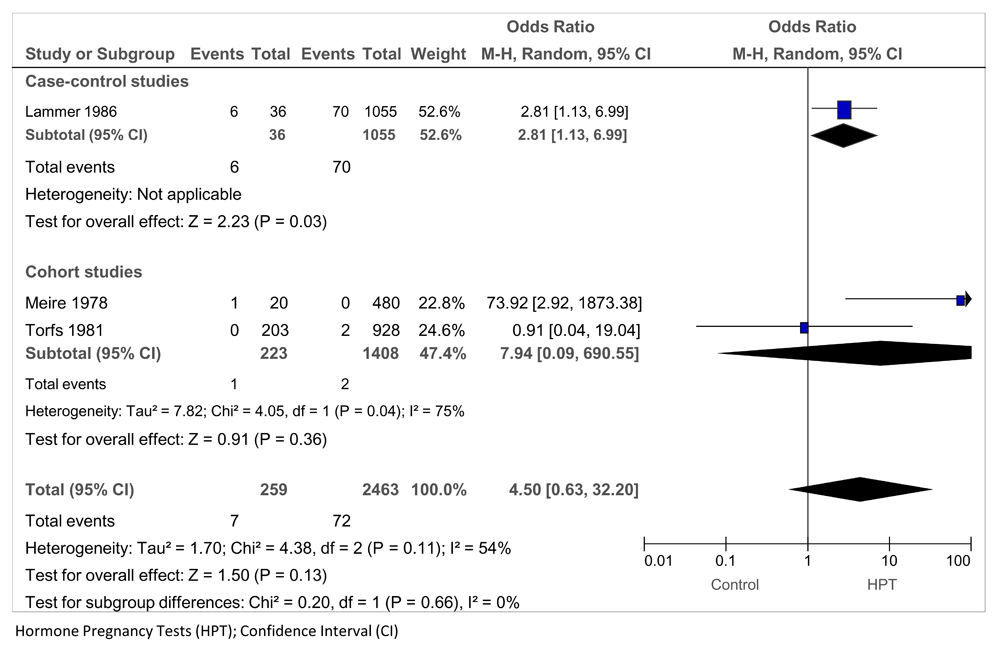
Gastrointestinal malformations and exposure to oral HPTs were reported in three studies: a case-control study (Lammer 1986) and two cohort studies (Meire 1978 and Torfs 1981), providing data on 2,722 mothers of infants, including 79 exposed to HPTs (Figure 5). The pooled OR = 4.50 (95% CI 0.63 to 32.20; P = 0.13; I2 = 54%). One case-control study (Polednak 1983) and one cohort study (Torfs 1981) examined the relationship between exposure to oral HPTs in pregnancy and urogenital malformations: pooled OR = 2.63 (95% CI 0.84 to 8.28; P = 0.10; I2 = 0%) (Figure 6).

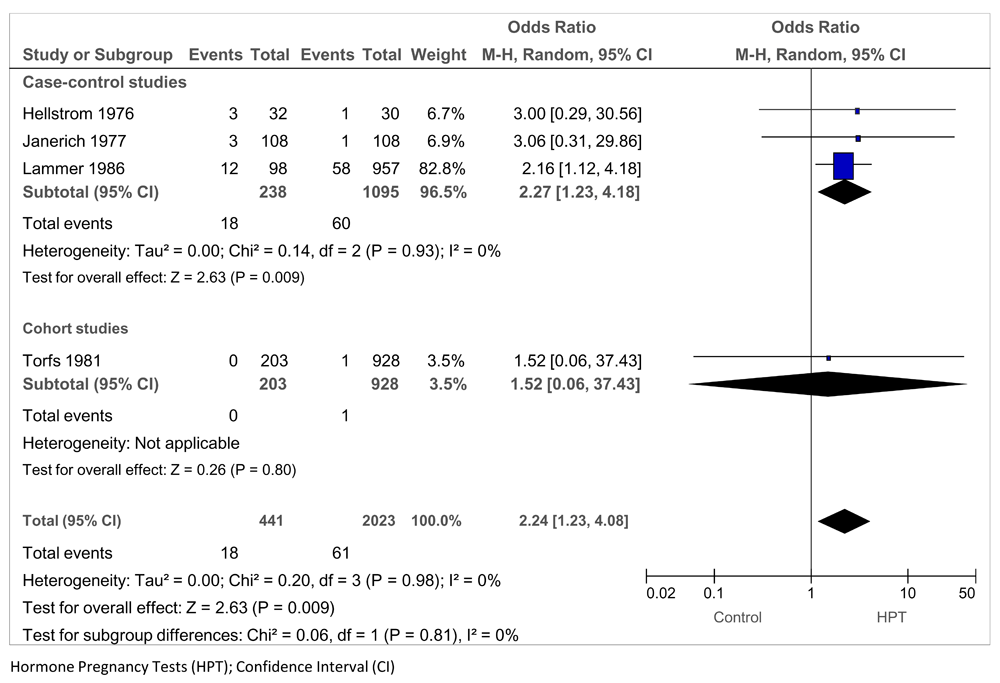
A relation between the exposure to oral HPTs and musculoskeletal malformations was reported in three studies: three case-control studies (Hellstrom 1976; Janerich 1977; Lammer 1986) and one cohort study (Torfs 1981) (Figure 7), based on 2,464 women, with 79 exposed to HPTs. The pooled OR = 2.24 (95% CI 1.23 to 4.08; P = 0.009; I2 = 0%). Removal of the zero study events (Torfs 1981) did not affect this result. The association of VACTERL with HPT exposure was reported in two case-control studies (Nora 1978-1 and Nora 1975), based on 135 women and infants and 27 exposed to HPTs; the OR was 7.57 (95% CI 2.92 to 19.07; P < 0.0001; I2 = 0%) (Figure 8).

We found 24 articles containing 26 studies that reported the association between exposure to oral hormone pregnancy tests in mothers and malformations in their infants: 16 were case-control studies and ten were prospective cohort studies. The overall quality of the evidence, assessed by the Newcastle–Ottawa Scale, was moderate.
We found significant associations for all congenital malformations pooled and separately for congenital heart malformations, nervous system malformations, musculoskeletal malformations, and the VACTERL syndrome. Many of these pooled analyses had zero heterogeneity, and the direction of effect favoured the controls in 30 of the 32 analyses undertaken (Torfs 81 provided the only effect estimate favouring HPT exposure). The analyses were also robust to sensitivity analyses, and there was no relation between NOS score and increasing risk.
Based on the assumptions that a teratogenic effect of HPTs would be mediated by actions on estrogen and progestogen receptors, and that concentrations of ethinylestradiol and norethisterone in the fetus would be too low to have a significant effect on those receptors, it has been suggested that there is no mechanistic argument for teratogenicity3. However, other unknown mechanisms might be at play. For example, Isabel Gal first reported concerns of malformations in the children of mothers exposed to HPTs in 196712, pointing out that bleeding often occurred in pregnant women soon after exposure and suggesting that that would affect the “equilibrium” of the uterus. Between 5 and 11% of exposed women had bleeding, and the RCGP survey reported induced abortions in about 10% of women13.
The drugs in Primodos were not tested for animal toxicity and teratogenicity at the time, which, although not unusual, meant that there was a gap in mechanistic understanding. A 2018 study showed that the components in Primodos are associated with dose-dependent and time-related damage in zebrafish embryos, and affect nerve outgrowth and blood vessel patterning in zebrafish12,14. Although it is difficult to compare drug actions between species, and evidence from animal studies is limited, the drugs accumulated in the zebrafish embryos, persisted for some time, and led to rapid embryonic damage12,14. In contrast, other animal studies have shown minimal effects on embryo development15. There is also evidence that estradiol and progestogens increase the expression of mRNA for isoforms of vascular endothelial growth factor (VEGF) in Ishikawa cells from human endometrial adenocarcinoma16.
Establishing causal associations in the absence of randomization can be difficult. However, the lack of randomized trials in our analysis should not be seen as a barrier to interpreting our findings. It would have been unethical to randomize individuals to drugs with known concerns, and randomization, like systematic reviews, was not the norm at the time. Furthermore, for questions about harms, the Oxford CEBM levels of evidence puts systematic reviews of case-control studies on a par with systematic reviews of randomized trials17.
However, observational methods have limitations18. First, interpretation can be affected by confounding factors. Although most of the studies in this review used matched controls, our analysis was based on raw data from the publications and did not adjust for confounders. Secondly, susceptibility bias can occur, as women with threatened abortions might be more likely to present and take the medication. Both of these problems can be mitigated by careful matching; 13 of the 16 studies controlled for the most important factor, item 5a on the NOS scale. Thirdly, the severity of malformations studied will have led to differing risk estimates across studies. Fourthly, inappropriate methods of ascertainment of the malformations and exposures could have introduced bias. Finally, incomplete and uneven reporting, along with publication bias (since it is likely that unreported studies exist) could introduce bias and alter the effect estimates.
The use of scoring systems to assess quality has been criticized. However, the NOS scale has been used widely in assessing the quality of non-randomized studies19–24. A NOS score between 0 and 9 has previously been used as a potential moderator in meta-regression25, and has been recommended by the Cochrane Collaboration26. A weakness of the NOS scale is the possible low agreement between assessors27. This was particularly the case when authors had limited experience in doing systematic reviews, but training, even of novices, improves agreement19.
The effects were also stable to sensitivity analyses, and changes in NOS score did not affect the risk estimates. The absence of subgroup differences between study designs for the risk estimates supports the robustness of the findings. We also tried to overcome publication bias by translation and assessment of unpublished data. The sample sizes in the studies for all congenital malformations, congenital heart disease, and nervous system malformations were sufficiently large to suggest that small unpublished studies would have little effect on the estimates unless they were highly heterogeneous. The analyses of gastrointestinal, urogenital, musculoskeletal, and VACTERL malformations were limited by their small sample sizes and low number of events: the interpretation of these effects should, therefore, be treated more cautiously. The significant effect observed for VACTERL should also be treated cautiously, as the confidence intervals for this effect were wide.
Our study has several strengths. We used standard systematic review methods, and by asking a focused question solely on exposure to HPTs, and excluding exposure to other hormones, we have been able to assess the heterogeneity of the effect estimates. However, as with any observational studies, there is always the possibility that an unknown confounder could be the cause of the observed difference. While such a possibility cannot be ruled out, the lack of heterogeneity means that such a confounder would potentially have to act in the same direction, despite many different confounders being collected and controlled for. Confounding factors with variable effects on the effect estimates would have probably led to a high degree of heterogeneity, which would have prevented pooling; this was not the case.
Regulators were first made aware of the link between exposure to HPTs and congenital malformations in 1967. After 1975, the Primodos label was changed to state that the medication should not be used in pregnancy because of a risk of malformations (see Figure 9). The evidence of an association has previously been deemed weak, and previous litigation and reviews have been inconclusive. However, we believe that this systematic review shows an association of oral HPTs with congenital malformations.
Our results show the benefit of undertaking systematic reviews, a study type not in routine use when most of these studies were done. For example, only one study (Greenberg 1997) out of nine reported a significant effect for all congenital malformations; the pooled estimate was significant. Much of the discussion over the associations of HPTs with congenital malformations at the time these studies were published focused on the lack of significance of individual studies28, although it was also recognized that the numbers involved were insufficient to reject the hypotheses29.
F1000Research: Dataset 1. Study extraction sheet, https://dx.doi.org/10.5256/f1000research.16758.d23349430
The Evidence Synthesis Working Group is funded by the National Institute for Health Research School for Primary Care Research (NIHR SPCR) [ProjectNumber 390]. The views expressed are those of the author(s) and not necessarily those of the NIHR, the NHS or the Department of Health
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary File 1. Committee on Safety of Medicines report 1975-colour pdf.
Supplementary File 2. Search Strategy.
Supplementary File 3. List of hormone pregnancy tests (HPTs) included in evidence search.
Supplementary File 4. Completed Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklist.
Supplementary File 5. Full references for included studies.
Supplementary File 6. Funnel Plots.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Evidence synthesis
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Britton H: Pregnancy Test. BMJ. 1956; 2 (4989). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: History of medicine
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 29 Jan 19 |
|||
|
Version 1 31 Oct 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Heneghan et al. also note that it has previously been argued that there is no known mechanism to explain teratogenicity of HPTs. Of course, the lack of a known mechanism does not mean that there is not one. It is well recognised that our knowledge of gene function and biological pathways is very incomplete. It is also very well recognised that response to drugs is heterogeneous - indeed, understanding genetics and heterogeneity of drug response is a major focus for the pharmaceutical industry today as it seeks to identify patient populations in whom new drugs are effective and safe.
This review and meta-analysis by Heneghan et al. is, therefore, a particularly important paper for our understanding of risks associated with HPT exposure including, as it does, 26 smaller studies combining to give sample sizes as large as 61,642 mothers with 3,274 exposed to HPTs during pregnancy.
The strength of the effects indicated by the Odds Ratios in these analyses, and the levels of statistical significance that are stated by the authors, surely now give pause for thought and reinforce the concerns that the previous studies were individually under-powered.
These results are robust across not only risk of "any" malformation (37% higher risk for children of mothers exposed to HPTs) but also risks of specific types of malformations, with risks doubling, tripling or higher compared to mothers not exposed to HPTs.
Of course, any meta-analysis of observational studies has its limitations and the authors discuss these, and their study design strategies to mitigate them, in detail. In the absence of large, randomised HPT trials (not only atypical of the era but also unethical in pregnant women) this meta-analysis is an essential and important step forward.
The authors' search strategy was designed to minimise the risk of missing published HPT studies. That 409 articles were found and only 24 (less than 6%) included after review underlines the breadth of the search.
The authors note that non-randomised study quality scoring systems such as the Newcastle-Ottawa Scale (NOS) have their critics but also note that the NOS scale has been widely used (from a simple Title/ Abstract search of PubMed, I see >1,200 citations in the last three years). Their methodology was rigorous to minimise assessor discrepancies using a standard review template, two assessors and resolving discrepancies in consultation with the other authors. They also note that a previous study showed scoring concordance which is improved by training and experience, even for novice assessors. Given the level of expertise of the authors, this bodes well for the study.
In their discussion the authors also consider the likely impact of other limitations such as confounding factors, susceptibility bias, ascertainment bias and publication bias.
Additional checks and balances during the statistical analyses sought to uncover any hidden biases or heterogeneity but did not indicate such issues and demonstrated that the results were robust to NOS score and to sensitivity analyses.
Following this detailed discussion of their work, the authors conclude that an association of oral HPTs with congenital malformations exists. This work must now be considered very seriously given the doubts that have been expressed regarding previous studies and the significance of these findings for the mothers who were exposed to HPTs during pregnancy and their children.
Heneghan et al. also note that it has previously been argued that there is no known mechanism to explain teratogenicity of HPTs. Of course, the lack of a known mechanism does not mean that there is not one. It is well recognised that our knowledge of gene function and biological pathways is very incomplete. It is also very well recognised that response to drugs is heterogeneous - indeed, understanding genetics and heterogeneity of drug response is a major focus for the pharmaceutical industry today as it seeks to identify patient populations in whom new drugs are effective and safe.
This review and meta-analysis by Heneghan et al. is, therefore, a particularly important paper for our understanding of risks associated with HPT exposure including, as it does, 26 smaller studies combining to give sample sizes as large as 61,642 mothers with 3,274 exposed to HPTs during pregnancy.
The strength of the effects indicated by the Odds Ratios in these analyses, and the levels of statistical significance that are stated by the authors, surely now give pause for thought and reinforce the concerns that the previous studies were individually under-powered.
These results are robust across not only risk of "any" malformation (37% higher risk for children of mothers exposed to HPTs) but also risks of specific types of malformations, with risks doubling, tripling or higher compared to mothers not exposed to HPTs.
Of course, any meta-analysis of observational studies has its limitations and the authors discuss these, and their study design strategies to mitigate them, in detail. In the absence of large, randomised HPT trials (not only atypical of the era but also unethical in pregnant women) this meta-analysis is an essential and important step forward.
The authors' search strategy was designed to minimise the risk of missing published HPT studies. That 409 articles were found and only 24 (less than 6%) included after review underlines the breadth of the search.
The authors note that non-randomised study quality scoring systems such as the Newcastle-Ottawa Scale (NOS) have their critics but also note that the NOS scale has been widely used (from a simple Title/ Abstract search of PubMed, I see >1,200 citations in the last three years). Their methodology was rigorous to minimise assessor discrepancies using a standard review template, two assessors and resolving discrepancies in consultation with the other authors. They also note that a previous study showed scoring concordance which is improved by training and experience, even for novice assessors. Given the level of expertise of the authors, this bodes well for the study.
In their discussion the authors also consider the likely impact of other limitations such as confounding factors, susceptibility bias, ascertainment bias and publication bias.
Additional checks and balances during the statistical analyses sought to uncover any hidden biases or heterogeneity but did not indicate such issues and demonstrated that the results were robust to NOS score and to sensitivity analyses.
Following this detailed discussion of their work, the authors conclude that an association of oral HPTs with congenital malformations exists. This work must now be considered very seriously given the doubts that have been expressed regarding previous studies and the significance of these findings for the mothers who were exposed to HPTs during pregnancy and their children.