Keywords
cotton, electroantennogram, Heliothis virescens, physiological state, Y-tube olfactometer
cotton, electroantennogram, Heliothis virescens, physiological state, Y-tube olfactometer
The new version includes minor changes suggested by one of the reviewers. Specifically, we have indicated that the same controlled conditions (temperature and humidity) used for insect rearing were maintained during bioassays.
See the authors' detailed response to the review by Andrea Clavijo McCormick
See the authors' detailed response to the review by Lukasz Stelinski
Parasitic wasps (parasitoids) use volatile organic compounds (VOCs) released by herbivore-infested plants as odor cues to locate their hosts1–4. However, the level of olfactory response to VOCs may not remain consistent throughout the duration of parasitoid adult life. Changes in physiological states can affect response to VOCs in parasitoids and other insects5–9. Olfactory plasticity in insects has been previously attributed to changes in physiological states such as age, mating, and nutritional status6,9–13.
Aging has been shown to affect olfaction in insects9,14–16. This effect may be associated with senescence of olfactory structures or processing units in insects. For instance, behavioral senescence of responses involved with locomotion, olfaction, and learning has been reported in fruit flies17–19. However, previous studies have reported mixed results regarding age-related plasticity of response to VOCs in parasitoids, thus the need for further studies14,20,21. Mating status of female parasitoids has also been reported to influence their foraging behavior and parasitization potential, with mated females showing a higher parasitization rate than unmated females13,22,23. However, the effect of mating on plasticity of parasitoid response to host-related plant volatiles has gained little attention despite its implications and relevance to host searching and parasitization potential (but see Chen & Fadamiro24).
In the present study, the relatively specialized larval endoparasitoid, Microplitis croceipes (Hymenoptera: Braconidae) and its caterpillar host, Heliothis virescens (Lepidoptera: Noctuidae) were used as a study system to test the effect of age and mating status on the olfactory response of parasitoids to host-related plant volatiles. Heliothis virescens is a generalist herbivore and a serious pest of cotton, tobacco, and other crops of economic importance25. Based on previous reports on olfactory response in M. croceipes to cotton volatiles26–30, four odor stimuli of varying complexity were chosen. These include: cis-3-hexenol (a green leaf volatile), α-pinene (a constitutive monoterpene), a 50/50 v/v binary mixture (cis-3-hexenol + α-pinene), and headspace volatiles from H. virescens-infested cotton. cis-3-Hexenol and α-pinene have been consistently detected in the headspace of H. virescens-infested cotton1,29,31 and have been reported to elicit antennal and behavioral responses in M. croceipes24,26,29,30.
Y-tube olfactometer was used to test attraction (behavioral response) of parasitoids while electroantennogram (EAG) was used to record antennal response of female M. croceipes to select host-related cotton volatiles. To the best of our knowledge, this is one of the few studies that investigated the effects of age and mating status on olfactory responses of parasitoids to host-related plant volatiles. The implications of these findings are discussed.
Cocoons of Microplitis croceipes were provided by the USDA-ARS, Insect Biology and Population Management Research Laboratory (Tifton, Georgia, USA) and reared in our laboratory (Auburn University, AL, USA) on 2nd – 3rd instar larvae of H. virescens. Upon emergence, adult wasps were transferred to aerated plastic BugDorm® cages (Megaview Science Co. Taichung, Taiwan) and supplied with 10% sucrose/water solution (w/v). Naive (untrained) parasitoids were used in both EAG and Y-tube olfactometer bioassays to test innate responses of parasitoids to host-related plant odors. Eggs of H. virescens were initially purchased from Benzon Research Inc. (Carlislie, PA, USA) and reared in our laboratory at Auburn University. Larvae of H. virescens were reared on pinto bean artificial diet according to Shorey and Hale32. In total, about 640 female parasitoids were used for bioassays. The general conditions for insect rearing and bioassays were 25 ± 1°C, 75 ± 5 % RH and L14:D10 h photoperiod.
Cotton (Gossypium hirsutum, var. max 9, All-Tex Seed Inc., Levelland, TX, USA) plants were grown in individual pots (9 cm high, 11 cm diameter) in a growth chamber at 26.6°C day, 25.6°C night, 60% RH, L16:D8 h (L:D) photoperiod. Seeds were planted in a top soil/vermiculate mixture. Plants used for headspace volatile collections were 4–6 weeks-old.
Upon emergence, parasitoids were separated based on their sex. Subsequently, an equal number of females were randomly designated to ‘mated’ or ‘unmated’ cages. Male parasitoids were put inside cages (19 × 13 × 10 cm) designated to mated females at a 2:1 (male: female) ratio while cages designated to unmated females contained females only. Female parasitoids were allowed to mate for at least 24 h before use in bioassays. Both mated and unmated females were further designated to separate cages based on their age. Age groups 1–3, 4–6 and 7–9 d-old were used in Y-tube olfactometer bioassays while age groups 1–3, 4–6, 7–9, and 10–12 d-old were used in electroantennogram (EAG) recording. The 10-12 d-old age group was not included in behavioral bioassays due to low numbers of insects surviving beyond 10 days in the laboratory, resulting in a low number of replicates. It should be noted that Y-tube olfactometer bioassays required more replicates than EAG recording.
Headspace volatiles were collected from H. virescens-infested cotton plants using the protocol described by Ngumbi et al.31. A plant pot with soil was wrapped with aluminum foil to minimize contamination. The plant was then placed in a volatile collection chamber (Analytical Research Systems, Inc., Gainesville, FL, USA) consisting of a 5-L air-tight glass jar. A purified air stream of 500 ml/min was passed through the jar at room temperature using Teflon tubing connected to an air delivery system. To induce volatiles from plants, 30 2nd –3rd instar larvae of H. virescens were allowed to feed on a cotton plant for 24 h during volatile collection from 1100 h one day to 1100 h of the following day. Headspace volatiles were collected with a trap containing 50 mg of Super-Q (CAT#: 2735, Alltech Associates, Deerfield, IL, USA) and eluted with 300 µl of methylene chloride. The resulting extract was stored in a freezer (-20°C) until use.
A Y-tube olfactometer (Analytical Research Systems, Inc., Gainesville, FL, USA) was used to test attraction of female M. croceipes to four odor stimuli of varying complexity. The setup and procedure was similar to that reported by Morawo and Fadamiro33. Parasitoids were introduced individually into the olfactometer and allowed to make a choice between test stimulus and control. Insects were tested once and discarded. Parasitoids that made no choice within 5 min were removed and excluded from the analyses. The number of non-responding parasitoids in the 24 sets of bioassays ranged from 0 to 5 with a mean of 1.2 insects per test.
cis-3-Hexenol (CAT#: W256307) and α-pinene (CAT#: 147524) (purity 95-99%; Sigma-Aldrich®, St. Louis, MO, USA) were individually formulated in hexane (HPLC-grade) at 1 µg/µl concentration. A 50/50 v/v binary mixture (cis-3-Hexenol + α-pinene) of the two compounds was also prepared. A central dose of 10 µg (10 µl sample) was previously determined to be optimal in a related study30. In separate bioassays, each compound or binary mixture was delivered as a 10 µl sample on filter paper strips (40 × 7 mm, CAT#: 1001090, Whatman® No. 1) in the treatment arm while the control arm contained the same volume of hexane (solvent control). Humidified and purified (charcoal filtered) air was pushed into each arm at the rate of 250 ml/min and removed by suction from the central arm of the olfactometer at the rate of 500 ml/min to avoid odor mix-up.
To test parasitoid response to H. virescens-infested cotton, one arm of the olfactometer was connected to an air-tight glass jar (5-L) containing an infested plant (with host larvae). The other arm was connected to a similar glass jar containing a pot of soil covered with aluminum foil, which served as control. A new plant was used on different days during which bioassays were conducted (5–7 plant replicates). Inlet air was pushed into the olfactometer through each jar at the rate of 300 ml/min and sucked out at the rate of 600 ml/min. Experiments were performed in a randomized complete block design with equal number of insect replicates from each age and mating status groups tested per day (n = 20 per test). All olfactometer bioassays were conducted between 1100 h and 1700 h on different days.
EAG response of female M. croceipes was recorded to measure odor perception in mated parasitoids. The EAG protocol used was previously described by Ngumbi et al.31. Glass capillaries (1.1 mm I.D.) filled with Ringer solution served as reference and recording electrodes. The reference electrode was connected to the back of the head of a female M. croceipes while the recording electrode was connected to the cut tip of the terminal segment of the antenna. The analog signal was detected through a probe (INR-II, Syntech, the Netherlands), and was captured and processed with a data acquisition controller (IDAC-4, Syntech, the Netherlands). EAG 2000 software v2.7 (Syntech, the Netherlands) was used to analyze digital signal readouts. Test stimuli were delivered as 10-µl samples (10 µg dose) on filter paper strips (7 × 40 mm) placed inside 14 cm Pasteur pipettes (Fisher Scientific, Pittsburgh, PA, USA).
Four treatment stimuli and two control stimuli were individually delivered as 0.2-s puffs of air, with 2 min interval between puffs. For each antennal preparation, the following stimuli were presented: hexane (control), methylene chloride (control), cis-3-hexenol, α-pinene, binary mixture, headspace volatile extract, hexane and methylene chloride. Thus, hexane and methylene chloride (solvent controls) were applied at the beginning and end of each recording series while the position of other test stimuli was randomized across replicates (see Morawo et al.34). Recordings were performed in a randomized complete block design with equal number of insect replicates (n = 10) from each age group tested per day.
Attraction of parasitoids to each of four test stimuli in Y-tube olfactometer was modeled as a binary response (stimulus = 1, control = 0) using logistic regression to analyze possible interactions between age and mating status factors. The model adequacy for each set of experiment was confirmed with a likelihood ratio test35. When no significant interaction was recorded, each factor was analyzed separately. For olfactometer data, deviation of parasitoid responses from a 50:50 (stimulus: control) distribution was analyzed using a Chi-square goodness-of-fit test. Absolute EAG responses (EAG response to solvent control deducted from EAG response to test stimuli) of mated parasitoids across age groups were compared using Kruskal-Wallis test. All analyses were performed in SAS v9.2 (SAS Institute Inc., Cary, NC, USA) with P = 0.05 level of significance.
Overall, there was no significant interaction between age and mating status factors for any of the four odor stimuli tested (cis-3-hexenol: P = 0.7295, Figure 1A; α-pinene: P = 0.7352, Figure 1B; binary mixture: P = 0.1136, Figure 1C; host-infested cotton: P = 0.7044, Figure 1D; Logistic Regression). However, mated parasitoids, 4-6 d-old were significantly (80/20%, χ2= 7.20, df = 1, P =0.0073) more attracted to the binary mixture (cis-3-hexenol + α-pinene) compared to hexane control (Figure 1C). Similarly, 1-3 d-old mated parasitoids were significantly (75/25%, χ2= 5.00, df = 1, P =0.0253) more attracted to H. virescens-infested cotton compared to control (Figure 1D). Parasitoids did not show significant attraction to test stimuli in other Y-tube olfactometer bioassays.

Bars represent percentage of mated and unmated parasitoids of ages 1–3, 4–6, and 7–9 d-old when given a choice between hexane (solvent control) and synthetic compounds cis-3-hexenol (A), α-pinene (B), a 50/50 v/v binary mixture of cis-3-hexenol and α-pinene (C), and a choice between control jar (with no plant) and Heliothis virescens-infested cotton (D). Synthetic compounds were formulated in hexane at 1 µg/µl and presented as 10 µl samples (10 µg dose). Thirty 2nd–3rd instar larvae of H. virescens were allowed to infest cotton plants for 24 h before bioassays. N = 20 responding parasitoids per choice test. Numbers in the bars indicate actual number of responding individuals that chose each arm of the olfactometer. Asterisk (*) indicates significant deviation of parasitoid responses from a 50:50 (stimulus: control) distribution (χ2 goodness of fit test, P < 0.05).
In general, the age of mated female M. croceipes did not have a significant effect on their EAG response to test odor stimuli (Figure 2). Both relatively young and older parasitoids showed similar levels of innate antennal sensitivity to single components, binary mixture and headspace volatile extract of host-infested cotton. These results are mostly in agreement with those recorded in Y-tube olfactometer bioassays.
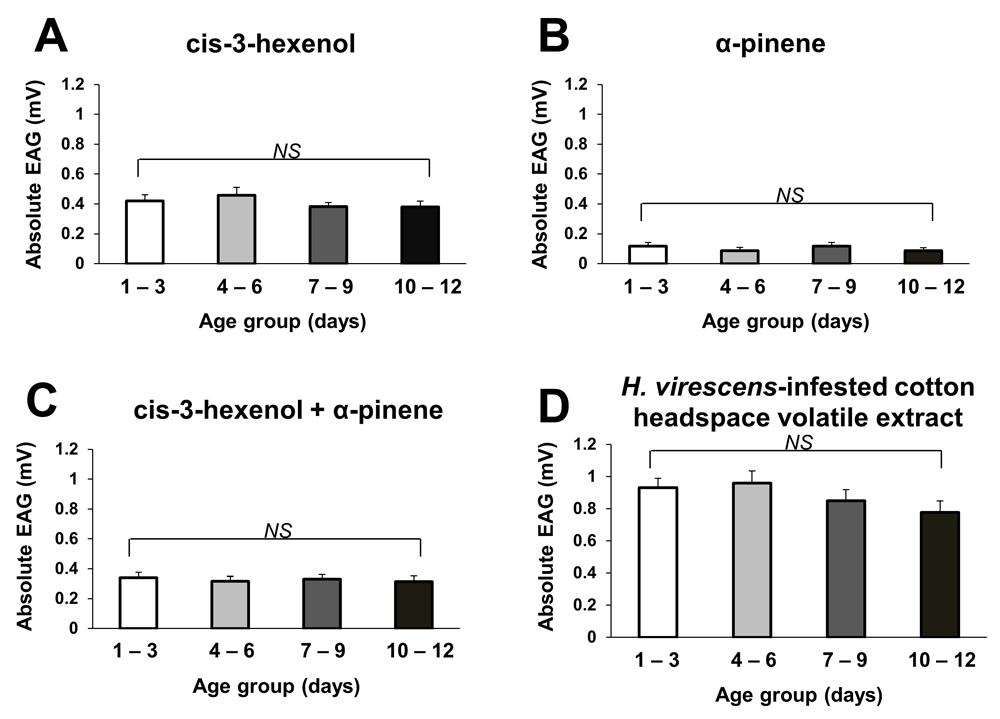
Bars represent mean absolute EAG responses (mV± SE, N = 10) of mated parasitoids age 1–3, 4–6, 7–9 and 10–12 d-old to cis-3-hexenol (A), α-pinene (B), a 50/50 v/v binary mixture of cis-3-hexenol and α-pinene (C), and Heliothis virescens-infested cotton headspace volatile extract (D). Absolute EAG for each stimulus is the actual EAG value minus EAG value of solvent control. Synthetic compounds were formulated in hexane at 1 µg/µl and presented as 10 µl samples (10 µg dose). NS indicates no significant difference.
Age and mating status are among several physiological factors that may affect olfactory responses of parasitoids to odor cues used in foraging. In the present study, neither age nor mating status of female M. croceipes played a major role in their olfactory responses to host-related plant volatiles. In general, relatively younger parasitoids showed similar levels of attraction to test stimuli as older parasitoids. Likewise, mated and unmated female parasitoids showed little or no difference in their attraction to host-related odors in Y-tube olfactometer bioassays. Subsequent experiments were conducted to measure the level of odorant perception in mated female parasitoids across age groups. The results showed that EAG response of mated parasitoids was not significantly different across age groups.
Although aging may negatively affect the host searching ability of female parasitoids, it may not always be attributed to a decline in odor perception in all species. Previous studies showed that relatively younger parasitoids tend to parasitize at a significantly higher rate than older parasitoids36–39. This may be in part due to senescence of some odorant processing apparatus or due to a decrease in energy levels in older parasitoids, thus affecting foraging activities. In a previous related study, age did not affect EAG response of M. croceipes to single VOCs21. In instances where age played a significant role, plasticity of olfactory response in insects at the peripheral and behavioral levels has been attributed to senescence through physiological and neuronal mechanisms17,19.
A few previous studies have reported that the mating status of female parasitoids may affect their foraging behavior and parasitization potential13,22,23,40. In the present study, mating status of female M. croceipes had no significant effect on their olfactory response to host-related odors. This suggests that both virgin and mated females of M. croceipes are likely to seek hosts using host-related odor cues. The concepts of haplodiploidy and optimal foraging provide a better understanding of the ecological ramifications of these results. Haplodiploid parasitoids such as M. croceipes produce male-only offspring from unfertilized eggs and male/female offspring from fertilized eggs. If the sex ratio of a local population is already at equilibrium, host foraging by unmated females may yield immediate benefits41. Otherwise, the cost may outweigh immediate benefits with the development of a male-biased population. Mated females are expected to optimize host foraging and produce progeny with a more balanced sex ratio, which is a critical fitness benefit for the population.
Overall, age and mating did not significantly affect the attraction of female M. croceipes to four test stimuli of varying complexity in the present study, with a few exceptions: mated parasitoids, 4–6 d-old showed significant attraction to the binary mixture and 1–3 d-old mated parasitoids showed significant attraction to H. virescens-infested cotton. The degree of host specificity in M. croceipes may provide a plausible explanation for the overall pattern and exceptions recorded in the present study. It has been proposed that specialist parasitoids exhibit strong congenitally fixed responses while generalist parasitoids exhibit greater plasticity of response to host-related cues42. This hypothesis is consistent with the present results in which M. croceipes (specialist) showed little olfactory plasticity with changes in physiological state. However, M. croceipes is not a strictly specialized species at the extreme of the spectrum. Instead, M. croceipes is a relatively specialized parasitoid utilizing Heliothis/ Helicoverpa host species43. This may possibly explain the few exceptions in which mated and relatively young parasitoids showed significant attraction to the binary mixture and host-infested cotton.
In summary, the current findings suggest that age and mating status do not play a major role in modulating olfactory responses of M. croceipes to host-related odors. Instead, plasticity of olfactory response may be limited in M. croceipes due to a strong innate sensitivity to host-related odor cues. This may have an impact on their potential as biological control agents. Other physiological factors such as level of nutrition may also have significant effect on olfactory plasticity in parasitoids11,44. This creates an opportunity for augmentation of parasitoids after field releases. Future studies, especially in the field, should investigate the effect of other physiological conditions that may affect plasticity of behavioral response to host-related odors in natural enemies.
The work presented here was part of an MS project completed by MB. The results presented here have been previously published as MB’s MS thesis available from Auburn University Electronic Theses and Dissertations repository: http://hdl.handle.net/10415/5108
F1000Research: Dataset 1. Attraction of female Microplitis croceipes to four host-related plant odors in Y-tube olfactometer bioassays, https://doi.org/10.5256/f1000research.16927.d22466945
F1000Research: Dataset 2. Electroantennogram (EAG) response of female Microplitis croceipes to four host-related plant odors, https://doi.org/10.5256/f1000research.16927.d22467046
This research was supported in part by the Alabama Agricultural Experiment Station.
The authors would like to thank Lindsay McMillan, Benjamin Reeves, and Savannah Duke for assistance with insect rearing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Chemical Ecology, Plant-Insect Interactions, Insect Behaviour, Plant Volatiles
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 19 Dec 18 |
||
|
Version 1 20 Nov 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)