Keywords
breast milk, influenza, infants, protection, transplacental, antibody
breast milk, influenza, infants, protection, transplacental, antibody
Sample collection details have been added: frozen foremilk collected in the morning, according to the study protocol always between hours 8 and 11, and 1-2 hours after the last feeding and not a convenience sample. Breast milk and serum samples were collected at 0, 1, 3, 6, 9, 12 months of lactation. However, a few samples were never received (missed visits) or samples have been used up in prior studies. Regarding decline in IgG, we agree that the levels decline throughout the lactation, as seen in Fig 2a. Due to the small number of samples, no statistical modeling or halflife calculations have been done.
The mPlex-Flu assay has been shown to strongly correlate with all functional assays of influenza specific antibodies, such as hemagglutination inhibition (HAI) assay and influenza virus micro-neutralization assay (Wang et al., 2018). We also used the HA specific bind blocking in multiple plex assay to confirmed the specificity of mPlex-Flu assay using human milk samples is similar to using human serum samples (New Supplementary Fig 1).
Multiplex assay: Dilutions were determined by pilot testing to ensure IgG and IgA influenza virus-specific antibody binding within the mPlex-Flu assay range, i.e. the detectable range of the mPlex-Flu assay (the lower to the upper limits of quantification (LLOQ and ULOQ)). The pilot testing had been performed to confirm that the dilution of samples enables all samples in this detectable range. Usually, the dilutions of samples are not laborious to be determined since mPlex-Flu can detect four Log10 scales.
Figure 1: We have increased the font on those figures. Log2 is customarily used for antibody titers to indicate fold-change. ‘Log2(netMFI)’ has been changed to ‘Log2(MFI)’ in the y-axis. ‘Log2(MFI +1)’ has been changed to ‘Log2MFI’ in the legend.
Several small typos have been corrected throughout.
See the authors' detailed response to the review by David C Dallas and Jiraporn Lueangsakulthai
See the authors' detailed response to the review by Kirsty Le Doare
The immune system in neonates and young infants is initially immature, without adequate protection against infections. The dogma is that during infancy, systemic protection is passively provided by transplacental transfer of IgG antibodies and oral and gastrointestinal mucosal protection via breast milk (BM) containing predominantly IgA and some IgG. However, the clinical protection provided by maternal influenza immunization or exposure varies by season and the corresponding match against circulating influenza strains. Therefore, maternal influenza exposure, whether through immunization or natural infection, provides maternal protection and has the potential to imprint the infant immune system and significantly impact infant morbidity and mortality, as recently comprehensively reviewed1.
Maternal influenza immunization prior to or during pregnancy provides clinical protection with 70% efficiency in the infant2–5. Only one study has described IgA antibody levels in human milk to influenza6. Their results suggested that vaccination using a single strain of influenza A (A/New Caledonia/20/1999, H1N1) induced significantly higher IgA antibody levels than those seen in non-vaccinated, and those antibodies were positively correlated with viral neutralization. In addition, higher rates of exclusive breastfeeding in the first 6 months of life were associated with protection against febrile respiratory illness in the infants of vaccinated mothers, suggesting mucosal protection against influenza by BM antibodies. However, there is little data on how influenza antibody levels, or strain-specific antibody profiles, vary between mother’s serum, BM and infant serum. Data on the kinetic changes in anti-influenza IgG profiles between mother-infant pairs are also largely lacking.
In this pilot study utilizing a novel multiplex assay, we assessed infant immunity to various influenza strains reflecting maternal anti-influenza IgG levels and profiles in serum, as well as characterized IgA antibody responses in breast milk, which are distinct and reflect mucosal immunity. This new analytical method was applied to a small number of samples showing feasibility and patterns suggestive that a larger study needs to be done to understand the impact of maternal imprinting on influenza immunity.
We utilized stored frozen human foremilk collected in the morning between hours 8 and 11 and 1–2 hours after the last feeding, from a prospective birth cohort recruited in 1997–2001 in Finland to assess immune factors in human milk7. As part of this study, breast milk and serum samples were collected during follow-up visits, and included colostrum and breast milk at 1 month, 3 months, 6 months, 9 months, and 12 months of duration of lactation. However, a few samples were never received due to missed visits or sample timing had to be moved due to illness or other difficulties in getting to the scheduled visits, and some samples have been used up in prior studies. In the present study, the earliest available BM and a paired, timed serum sample was assessed from each of 7 mothers; ranging from 3 days to 2 months post-partum. Paired infant serum samples were assayed at up to three time points during the first 12 months of life, one prior to assumed disappearance of transplacentally transferred IgG, and one after. The samples collected in this cohort have been stored at -80°C with no recurrent freeze-thaw cycles. Aliquots have successfully been used in the past for measurement of serum and BM antibody levels with good antibody levels detected both for IgG and IgA7. These mothers were unvaccinated, as guidelines for maternal influenza immunization were not in place at the time samples were collected. None of the infants had been vaccinated to influenza. Clinical characteristics and timing of samples available are shown in Table 1. The study was approved by the institutional review boards of the Helsinki University Central Hospital, the City of Helsinki, and the University of Rochester Medical Center, Rochester, NY.
We have developed a multiplex assay (mPlex-Flu) that simultaneously measures absolute antibody concentrations against up to 50 influenza strains8. The mPlex-Flu assay has several advantages over the traditional hemagglutinin inhibition (HAI) titer assay: a linear readout over 4 logs, and high sensitivity. Our previous studies also showed that mPlex-Flu assay results highly consistent with the results from HAI and ELISA assays in human pre-and post-influenza vaccine study8. In the present study, a panel of 30 individual recombinant hemagglutinin (rHA) proteins of influenza virus strains and chimeric rHAs were used (see Table 2). This allowed us to estimate the specific anti-influenza IgG and IgA levels against H1, H2, H3 and Flu B seasonal influenza strains, as well as HA stalk specific antibodies using chimeric rHA (i.e. head from one influenza strain and stalk from another strain), cH5/1 and cH9/1 specific for group 1 (i.e. H1, H2, H5, H6) and cH4/7 and cH5/3 for group 2 (i.e. H3, H7) influenza strains, as previously described8. The mPlex-Flu assay has been shown to strongly correlate with functional assays of influenza specific antibodies, including the hemagglutination inhibition (HAI) and influenza virus micro-neutralization assays8,9. In addition, we have previously shown that the mPlex-Flu assay has excellent strain specificity using competitive binding studies8, which we also used here to confirm our results in human breast milk samples (Supplementary Figure 1). These data suggest minimal non-specific binding in paired samples. As we and others have previously demonstrated, there is cross-reactivity of anti-HA antibodies between antigenically similar influenza strains based on similar or shared epitopes.
Seasonal Vaccine strains in Bold
Different colors determine the influenza virus type or subtypes. Influenza B virus showed in Green. Bark blue, gray, and light blue, purple and dark green present H1, H2, H5, H6 and H9 subtype influenza viruses, respectively, HA phylogenic group 1 viruses. Red and orange present H3 and H7, HA phylogenic group 2 viruses. Other black present the HA1 domains of HA or chimeric HAs.
In the present study, samples of maternal serum (diluted 1:500 for IgA and 1:5000 for IgG), infant serum (1:10) and BM (1:10) were diluted using PBS and incubated with rHA coupled Luminex beads (Luminex Corp, Austin, TX). IgG or IgA binding was detected with anti-human IgG or IgA specific secondary antibodies (SouthernBiotech, AL, Cat No 2040-09, 2050-09, respectively). Median fluorescence intensities (MFI) were measured using a MAGPIX multiplex reader (Luminex Co.,TX) and converted into absolute IgG concentrations (ng/mL) using a IgG standard curve generated with a human standard serum, a mixture of sera from four subjects containing high levels of anti-influenza HA IgG and IgA against multiple influenza strains8. Since serum IgA is monomeric, while BM secreted IgA (SIgA) is dimeric10, the standard curves of BM SIgA against influenza viruses are very different from that of serum standard curves generated from our human standard serum sample. We thus report the magnitude of BM IgA anti-influenza HA antibody levels in MFI units. For consistency, and to allow direct comparison, we also report IgG levels in MFI units. All data were analysis by Prism 7, and heatmap figures were generated by Mathematic 11.2.
This new analytical method was applied to a small number of samples showing feasibility and several interesting patterns. BM had a pattern of IgG reactivity very similar to maternal serum. Also, the levels and strain reactivity patterns of anti-influenza IgG in mother’s serum matched that of her infant, suggesting a robust transplacental transfer of antibodies. As expected, there was a steady decay of infant influenza specific IgG levels by 6 to 8 months of age (Figure 1A). This decay was, however, not comparable in all infants. Interestingly, mothers with highest anti-influenza HA IgG antibodies had infants with high initial anti-HA antibody maintained until 6 months of age (Pairs #3 and #7), compared to a mother with the lowest initial IgG (Pair #4), suggesting that initial levels attained transplacentally are directly associated with the rate of decline of passive systemic immunity. By the end of the first year, infant #1 maintained 6-month IgG antibody levels, which is likely due to new, natural exposure. Supplementary Figure 2 shows the heatmaps of IgG and IgA antibodies to influenza strains measured by multiplex array in paired mother’s serum (MS), breast milk (BM) and infant serum (IS) samples. Figure 2A shows the trajectory of IgG antibodies to selected individual strains.
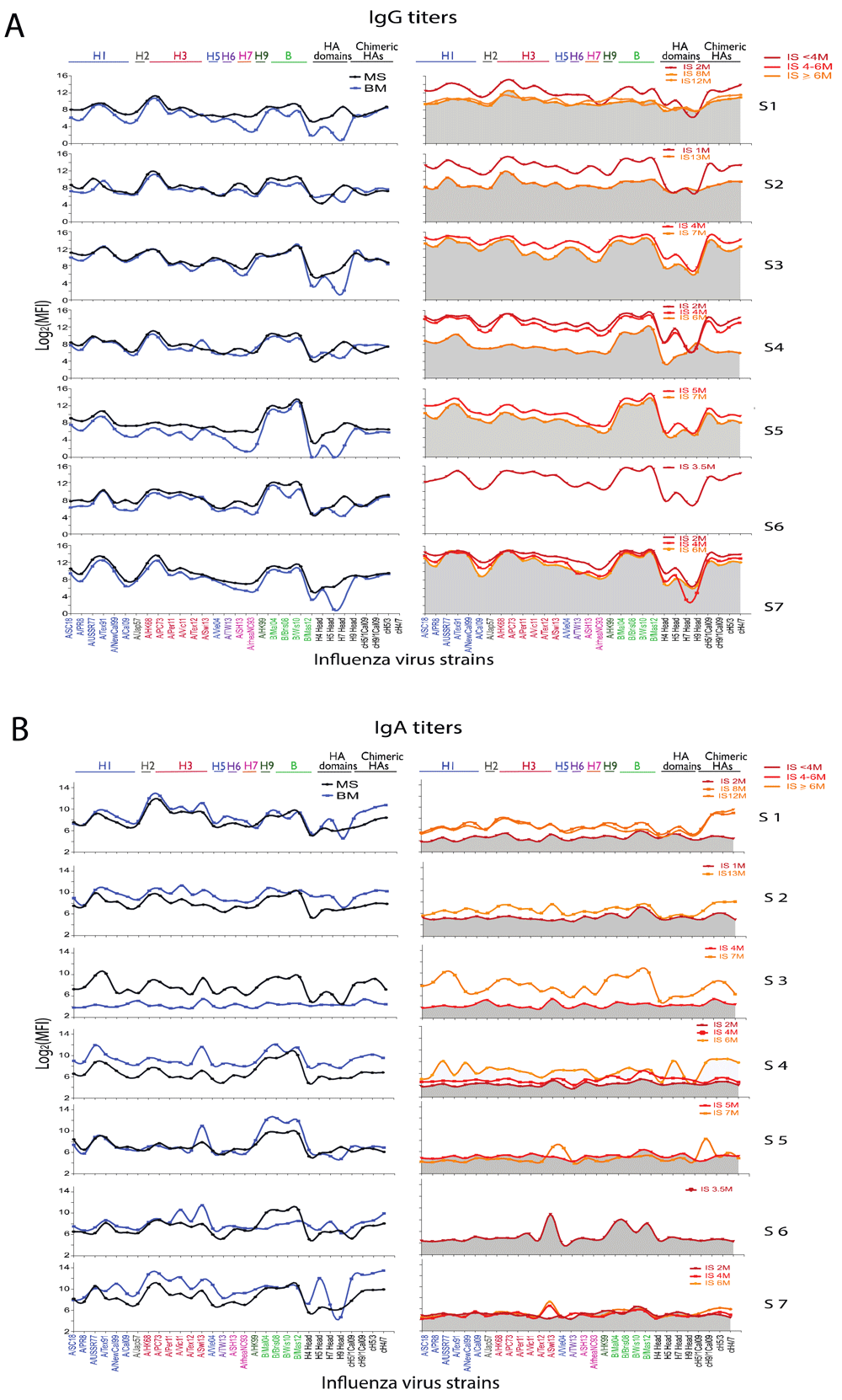
The antibody levels against homologue and cross-reactive HA proteins from different influenza virus strains were evaluated by the mPlex-Flu assay using paired mother’s serum (MS), breast milk (BM) and infant’s serum (IS) at different ages expressed as months. A. The IgG antibodies against individual HA of influenza virus strains. Maternal serum was diluted 1:5000, infant serum 1:10 and breast milk 1:10. The IgG antibodies against influenza virus HA were estimated using Phycoerythrin (PE)-conjugated anti-human IgG (γ chain specific) secondary antibodies (SouthernBiotech, AL) and shown as means of median fluorescence intensity (MFI) (n=3). The antibody titers (Log2MFI) against individual rHA of influenza virus strains were plotted and connected by LOWESS curves. In the panel of IgG MFI units of infant serum samples, the gray is the area under HA antibody curve of oldest sampling time point in the same subject. B. The IgA antibodies against individual HA of influenza virus strains. Maternal serum was diluted 1:500, infant serum 1:10 and breast milk 1:10. Then IgA antibodies were detected using PE-conjugated anti-human IgA (α chain specific) secondary antibodies (SouthernBiotech, AL) and shown as the mean of median fluorescence intensity (MFI) (n=3). The antibody titers (Log2MFI) against individual rHA of influenza virus strains were plotted and connected by LOWESS curves. In the panel of Ig MFI units of infant serum samples, the gray is the area under the HA antibody curve of the youngest time point in the same subject.
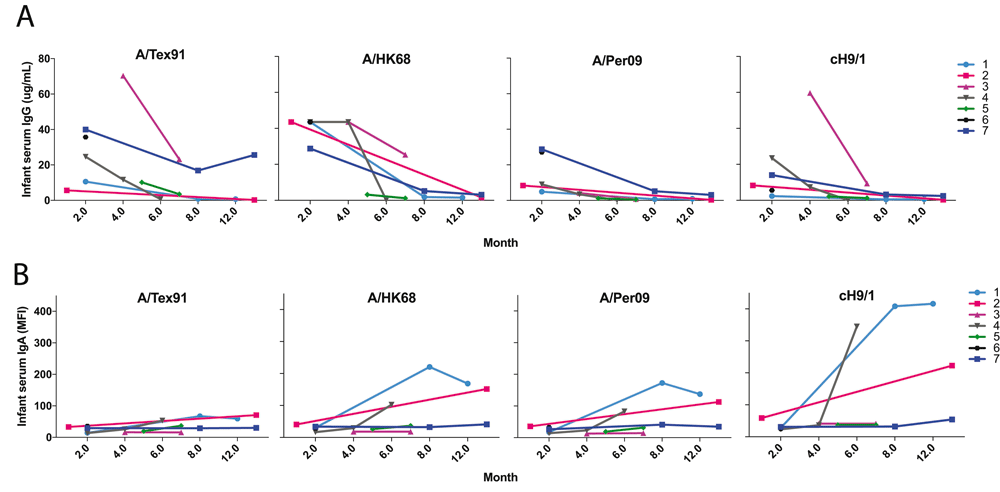
A. IgG antibody levels against selected influenza virus HA were plotted over time during first year (infant age in months) for 7 infant subjects from Figure 1A data. The specific antibody concentration of IgG is shown as the means for triplicates (n=3). B. The IgA antibody levels against selected influenza virus HA in infant sera were plotted over time during first year for 7 infant subjects from Figure 1B data. The specific antibody levels of IgA are shown as the means for MFI units (n=3).
Unlike with IgG antibodies, BM influenza virus HA-specific IgA antibody levels and patterns differed from serum IgA reactivity patterns, when visually comparing the pattern of antibody reactivity. Only three mother-infant pairs showed high a degree of concordance (Pairs #1, #4, and #5). This may be due to the mucosal homing of IgA producing antibody-secreting cells to the mammary gland, resulting in a different antibody profile in breast milk from serum. Very low serum IgA antibodies in infants are consistent with the fact that IgA does not cross the placenta (Figure 1B). The pattern of IgA anti-HA antibody binding was largely similar to that of mother’s serum and milk IgG, and predominantly against H1, H3 and B influenza strains. As opposed to infant IgG responses, most of the infants (Pairs #1–4) showed an increase in IgA responses throughout the first year of life (Figure 1B), whereas no matching IgG antibody response was seen. This may be due to natural, mucosal exposure to influenza inducing local responses, possibly in the absence of a systemic infection inducing IgG antibodies. Figure 2B shows the trajectory of IgA antibodies to a few individual strains. Both anti-stalk group 1 (cH5/1, cH9/1) and group 2 (cH5/3 and cH4/7) IgG (Figure 1A) and IgA antibodies (Figure 1B), which can confer cross-strain immunity, were abundant in the early months in infant serum and BM, respectively.
Our pilot data suggest feasibility for measuring antibody responses to influenza strains in maternal breast milk and paired infant serum utilizing a novel multiplex assay to assess changes over time. We show an anticipated decline in IgG responses to influenza HA in the first 5 months of life reflecting waning passive transplacentally acquired immunity, whereas the systemic IgA response in infants appears to be relatively poor, consistent with no vertical transfer. This does not exclude the possibility that such young infants might have a response at mucosal surfaces, such as in saliva upon exposure. Throughout the first year, however, a small increase in IgA antibodies, but not IgG antibodies, is seen in these unvaccinated infants, possibly suggesting that the adaptive immune response to natural exposure, in the absence of systemic infection induces initially local IgA, but not IgG antibodies. We also show that while the IgG specificity patterns were rather similar between breast milk and maternal serum, the patterns for IgA specificity were distinct and more pronounced in BM than those seen in serum. These data suggest that during this time, breast milk IgA may indeed be an important means of providing mucosal protection, which closely reflects maternal mucosal exposure to a variety of influenza strains to benefit the infant. Our previous results comparing food-specific IgA in human milk and maternal serum have shown similarly marked differences between human milk and serum IgA antibody profiles7. This is likely reflecting the fact that IgA-producing cells in mammary gland originate in the gut- and bronchus-associated lymphoid tissue, which constitute an important defense mechanism of the newborn11.
Although our study does not address the antibody profiles in vaccinated dyads, maternal influenza vaccination is recommended during pregnancy to induce infant post-partum passive immunity, and for infants after 6 months of age, although many choose to defer vaccination. As indicated by our pilot study, responses in mothers and infants are heterogeneous. At present, there is no robust literature or clinical method to optimize the vertical transfer of protective antibodies. Furthermore, the mechanisms of imprinting or maternal imprinting of the infant immune system are incompletely understood. Thus, there is a critical need for empirical data regarding maternal (serum, BM) and infant (serum) influenza-specific antibody levels over time to inform about maternal impact of influenza immunity, and to predict the individual window of infant susceptibility to influenza. Larger studies are required to further elucidate the interesting findings of this pilot study to aid in assessment of (maternal) imprinting of influenza immunity. Knowing the scope of passive immunity, both transplacental and that provided by BM, and when it vanishes, would allow for precision maternal-fetal and infant vaccination schedule design, also accounting for circulating influenza strains, seasonality, and vaccination status.
F1000Research: Dataset 1. Raw data for the present study, https://doi.org/10.5256/f1000research.16717.d22413712, including the following files:
Influenza-specific IgA antibody data as MFI. The file contains the IgA antibody data expressed as MFI for a panel influenza strains generated by mPlex-Flu assay utilizing all breast milk and serum samples. (IgA_20160908_MFI.xlsx)
Influenza-specific IgG antibody data as MFI. The file contains the IgG antibody data expressed as MFI for a panel influenza strains generated by mPlex-Flu assay utilizing all breast milk and serum samples. (IgG_20160908_MFI.xlsx)
Comparison of influenza-specific IgA antibodies between paired samples. The MFI titer comparison of IgA antibody of maternal serum (MS) vs breast milk (BM) and infant’s serum (IS) over time using the Prism 7 software. (IgA version2018.pzfx)
Comparison of influenza-specific IgG antibodies between paired samples. The file contains MFI unit comparison of influenza-specific IgG antibodies of maternal serum (MS) vs breast milk (BM) and infant’s serum (IS) over time using the Prism 7 software. (IgG version2018.pzfx)
Program code for IgA heatmap. The Mathematica 2 program code for generation of the heatmap figure of IgA data of maternal serum (MS), breast milk (BM) and infant’s serum (IS) from mPlex-Flu assay. (IgA MFI Revised.nb)
Program code for IgG heatmap. The Mathematica 2 program code for generation of the heatmap figure of IgG data of maternal serum (MS), breast milk (BM) and infant’s serum (IS) from mPlex-Flu assay. (IgG MFI Revised.nb)
The project described was supported by Grant Number K08 AI091655 (KMJ), R21 AI138500 (MZ, JW) and R01AI129518 (MZ) from the National Institute of Allergy and Infectious Diseases, and UL1 TR002001 (MZ, JW) from the National Institute for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institute for Advancing Translational Sciences, or the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Figure 1. The specificity of mPlex-Flu assay for determination of IgG/IgA antibodies against influenza viruses in human breast milk samples. The specificity of mPlex-Flu assay determined by rHA protein pre-adsorption. The milk IgG or IgA antibody binding with/without rHA pre-absorption in mPlex-Flu assay is shown. The mean binding (MFI) showed in heatmap (n=4). We determine the specificity of mPlex-assay by using the antibody pre-adsorption assay as we previously descripted8. Briefly, breast milk samples (1:10 dilution, within the detectable range of mPlex assay) were pre-incubated with individual recombinant HA (rHA) protein of influenza virus 0.25 μg for 1 hour for pre-absorption of the HA specific antibodies in breast milk; then run the mPlex-Flu assay to show the antibody binding with/without pre-absorption of A/Texas/36/1991 (A/Tex91, H1), A/Victoria/361/2011 (A/Vic11, H3), B/ B/Massachusetts/2/2012 (B/Mass12, B influenza), respectively. The results showed Pre-absorption with rHAs of A/Vic11 significantly inhibited two H3 strains in IgA, and all 5 H3 strains in IgG testing of breast milk samples. Those H3 strains are antigenic similar to A/Vic11, but did not affect IgG and IgA antibody binding with other influenza virus strains. Similar results were observed when milk samples were pre-absorbed by rHA of B/Mass12 and A/Tex91. Those results suggested a high type or subtype specificity of the mPlex-Flu assay for determination of human breast milk IgA and IgG anti-influenza strain specificity.
Supplementary Figure 2. Heatmaps of IgG and IgA antibodies to influenza strains measure by multiplex array in paired mother’s serum (MS), breast milk, (BM) and infant serum (IS) samples. The antibody levels against homologue and cross-reactive HA proteins from different influenza virus strains were evaluated by the mPlex-Flu assay using paired mother’s serum, breast milk (BM) and infant’s serum at different ages expressed as months. A. The IgG antibodies against individual HA of influenza virus strains. MS samples were diluted 1:5000, BM samples were diluted 1:10, and IS samples were diluted 1:10. The IgG antibodies against influenza virus HA were estimated using Phycoerythrin (PE)-conjugated anti-human IgG (γ chain specific) secondary antibodies (SouthernBiotech, AL) and shown as mean median fluorescence intensity (MFI) (n=3). B. The IgA antibodies against individual HA of influenza virus strains. MS samples were diluted 1:500, BM samples were diluted 1:10 and IS samples were 1:10 diluted. Then IgA antibodies were detected using PE-conjugated anti-human IgA (α chain specific) secondary antibodies (SouthernBiotech, AL) and shown as MFI unit also (n=3).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vaccine immunity
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: milk protein digestion and peptidomics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vaccine immunity
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 11 Mar 19 |
read | |
|
Version 1 20 Nov 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)