Keywords
wing pattern, butterflies, moths, sulfated polysaccharides, evolution of development
wing pattern, butterflies, moths, sulfated polysaccharides, evolution of development
The wing patterns of butterflies and moths are not only physical characteristics that interact with their surroundings according to the laws of physics, such as through the absorption or reflection of heat; the spectacular array and the subtle variations in wing patterns found among ca. 160,000 Lepidoptera species are due to the fact that these patterns are also ornaments, and as such, are there to interact with an observer—whoever that observer may be—contributing not only to natural but also to sexual selection. Hence, understanding how these ornaments evolve and how they are regulated during their development would help understand the process of selection. Altering the expression of a gene ligand, like Wnt, through injecting heparin at the crucial stages of wing pattern development, can provide a glimpse into the mechanisms underlying wing pattern evolution and development.
Since pioneering experiments on the common buckeye, it has been known that the wing pattern of a butterfly can be altered by heparin injection during the pupal stage (Serfas & Carroll, 2005). When, in 2017, I injected heparin into two prepupae, along with several pupae, of the io moth, it was a deviation from standard practice, which called for targeting early pupal stages. In that experiment, the survival rate of the injected prepupae was 100%, while for the pupae, it was 8%, while the two groups achieved similar pattern transformations (Sourakov, 2017). This, combined with the curiosity of how other species would be transformed by heparin injections, prompted me to conduct the experiments described below.
The common buckeye, Junonia coenia—a nymphalid species on pupae of which heparin-induced wing pattern manipulation was already thoroughly explored by Serfas & Carroll (2005) —offered a good model for examining whether injection at the prepupal stage resulted in different transformations. Additionally, I conducted experiments on several other Lepidoptera species from several families: Nymphalidae: the gulf fritillary (Agraulis vanillae) and the tawny emperor (Asterocampa clyton); Papilionidae: the giant swallowtail (Heraclides cresphontes), the spicebush swallowtail (Pterourus troilus), and the zebra swallowtail (Eurytides marcellus); Erebidae: the leopard moth (Hypercompe scribonia), the fall webworm (Hyphantria cunea) and the acrea moth (Estigmene acrea); and Crambidae: the mulberry leaftier (Glyphodes sibillalis). All of these are highly patterned species common to Florida. Collectively, they offer a good basis for comparison as they represent several different Lepidoptera lineages.
Several hundred caterpillars of all species used in this study were collected in Gainesville, Florida, and raised to pupation, with 275 of them used as experimental groups (see Table 1 for numbers per species). Additionally, several unmanipulated individuals were reared in the same conditions for each species as controls. Caterpillars were then reared inside plastic bags or containers on the foliage of their respective hostplants as follows: on Plantago lanceolata (buckeye); on Passiflora incarnata (gulf fritillary); on Celtis laevigata (tawny emperor); on Zanthoxylum clava-herculis and Z. fagara (giant swallowtail); on Cinnamomum camphora and Sassafras albidum (spicebush swallowtail); on Asimina triloba (zebra swallowtail); on Tradescantia ohiensis and Melilotus alba (acrea and leopard moths); on Juglans nigra (fall webworm) on Morus sp. (mulberry leaftier moth). The buckeyes were reared at natural light condition at 27°C; all others, at 24 hour light in the lab at 23°C. The immature stages used to generate the graph in Figure 8 were weighted using Metter Toledo AL104 analytical balances.
Heparin injections were made with either a 10-µl Hamilton syringe or a 0.3-ml hypodermic syringe. In the latter case, the amount was measured out with the 10-µl syringe. The heparin solution was made from porcine-derived heparin sodium salt (manufactured by MP Biomedicals, Inc., supplied by Fisher Scientific) dissolved in distilled water. Different concentrations and volumes of heparin solution were injected. Concentrations and volumes used were different for each species (according to their size), and were sometimes varied in order to achieve variation in response (Table S1) (Sourakov, 2018). Experiments also investigated the effect of temperature on an individual’s response to injection (Table S1) (Sourakov, 2018). When the temperature was lowered from 23°C to 16°C, the experimental animals were placed in this temperature 1-3 hours prior to injection and left there for at least 24 hours afterwards. If a prepupa was injected at this temperature, it was allowed to pupate at 16°C and left an additional 24 hours as pupa at 16°C before being returned to 23°C. For many individuals, the exact time from injection to or after pupation was determined using time-lapse photography with precision of 0.5-1 hour, while for others it was only determined approximately (Table S1) (Sourakov, 2018). While the numbers of control individuals varied depending on availability, from 5 (in case of mulberry leaftiers) to 32 (in case of buckeyes) they were also supplemented by consulting the MGCL collection holdings for these species from the Gainesville area.
Common buckeye, Junonia coenia. In buckeyes, the heparin-induced changes consisted of the orange parafocal element being lost as the distal marginal and submarginal bands shift basally and overrun it. The width and clarity of the normally cream-colored markings on the dorsal forewing surface is reduced, along with the reduction of the forewing eyespots. This resulted in specimens that are overall less colorful dorsally (Figure 1Ai–Li). Ventrally, the same individuals exhibited a reduction in the size of eyespots and the diffusion of some of the wing pattern elements (Figure 1Aii–Lii). While buckeyes are quite variable in nature, the three “control” groups (non-manipulated, H2O-injected, and wild adults collected from the same locality as caterpillars) were similar to each other. The experimental heparin-injected group, on the other hand, ranged from displaying phenotypes that were not found in any of the control groups (as in Figure 1G–L) to presenting normal coloration (Figure S1) (Sourakov, 2018). An almost complete modification of the pattern as shown in Figure 1G occurred only once and this individual, although it made it out of the pupa, did not appear viable, unable to harden its wings, climb, or fly properly.
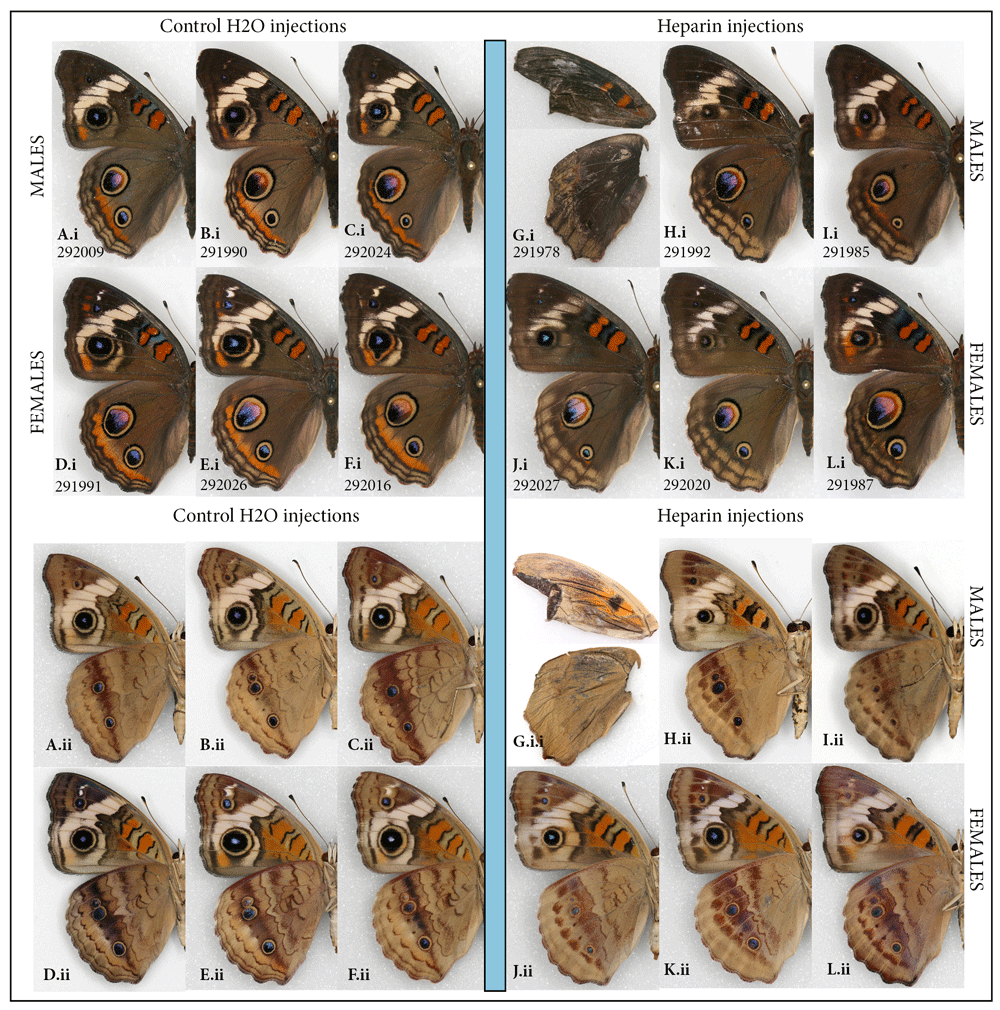
Heparin-induced wing pattern changes (right) vs. controls (left) on the dorsal (i) and ventral (ii) surfaces of the common buckeye, Junonia coenia. (A–F) Control group: (A–C) males and (D–F) females injected H2O as prepupae. (G–L) Experimental group: (G–I) Males and (J–L) females injected heparin as prepupae. For both groups, specimens shown are the ones in which the dorsal orange hindwing band was expressed the least. See Table S1 for injection details and Figure S1 for more individuals from both groups.
Analysis of the wing pattern of all surviving individuals injected as prepupae with heparin (N=21) suggests that injection at the early prepupal stage (as defined in Extended Data) (Sourakov, 2018) is more likely to lead to survival and transformation: among 10 experimental individuals sporting the most transformed wing patterns, three were injected as late prepupae and seven as early prepupae.
The taxonomy of the genus Junonia in the New World has been complicated by the variability in wing patterns found within and among species and populations. For the widespread species J. coenia this has led to the generation of various taxonomic hypotheses, and modern approaches have recently been used to test them (e.g., Peters & Marcus, 2017). Laboratory-generated aberrations, such as the ones figured in the present study, may be useful for understanding the source of variability found among natural populations and perhaps even help in making taxonomic decisions.
The Tiger moths: the leopard moth, Hypercompe scribonia, the acrea moth, Estigmene acrea, and the fall webworm, Hyphantria cunea. Among 36 individuals of the leopard and acrea moths that survived injection at different stages of development, transformation was restricted to a single individual in each species injected as a prepupa within 12 hours before pupation (HBP), despite the fact that doses and concentrations injected were quite high and timing varied widely.
The heparin-induced changes were similar in their manner (Figure 2). The alterations consisted of a very noticeable elongation of the black markings, to the point where some of the normally distinct spots sometimes merged, which happened both dorsally and ventrally. In the leopard moth, the expansion of individual spots was not consistent throughout the forewing, but was restricted to areas of the forewing adjacent to the discal spot, with spots along wing margins remaining unchanged (Figure 2C). Hindwing markings also expanded in response to heparin, especially in the acrea moth (Figure 2B). I examined hundreds of individuals of both species in the collection of the McGuire Center (MGCL) but did not find any with similar phenotypes.
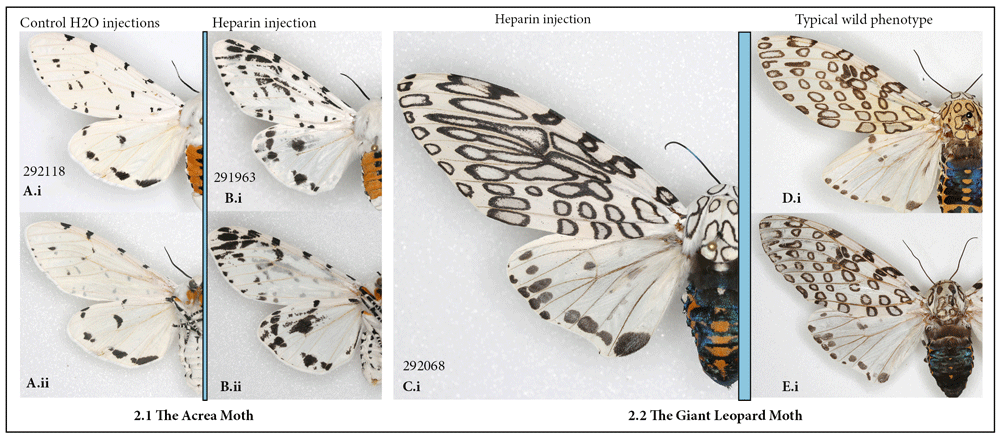
(A, B) The acrea moth Estigmene acrea: (A) control; (B) injected heparin as prepupa (i) dorsal (ii) ventral. (C–E) The leopard moth, Hypercompe scribonia, dorsal view: (C) injected heparin as prepupa; (D–E) typical wild-collected specimens. See Table S1 for injection details.
For the fall webworm, for which the caterpillars of the entirely white-winged form were available, the experimental individuals that emerged (N=12) were identical to controls (N=40), despite the injection of prepupal and early pupal stages.
The gulf fritillary, Agraulis vanillae. The gulf fritillary has been one of several model organisms in recent investigations into wing pattern formation (Martin & Reed, 2014; Mazo-Vargas et al., 2017). Martin & Reed (2014, p. 376) showed how heparin injections result in the expansion of Wnt-positive patterns and the reduction or loss of Wnt-negative patterns in this species. This species provided a convenient subject for observing a gradual change in pattern, enabling testing of which developmental stage and temperature are most sensitive to treatment, leading to greater modification of wing pattern. A total of 11 successful manipulations, arranged in order of magnitude of effect on wing pattern (Figure 3Bi), can be correlated with injection stage and dose by consulting Table S1 (Sourakov, 2018). This wing transformation spectrum is contrasted with normal wing pattern that was not altered by injection at 1HBP (Figure 3A), or by pupation at colder temperature (Figure 3M).

Heparin-induced wing pattern changes in the gulf fritillary, Agraulis vanillae (A–L) vs. rare natural aberrations from the MGCL collection (N–O), and control (M); (i) dorsal, (ii) ventral surfaces. See Table S1 for specimen details.
Some of the transformed wing patterns (e.g., Figure 3C, I) are very similar to those of two wild-collected aberrant individuals, which were discovered in the MGCL collection after examining approximately 1000 pinned specimens (Figure 3N–O). These aberrations, while rare, may be of significance for understanding selection mechanisms that lead to divergence of wing patterns in Heliconiinae.
The tawny emperor, Asterocampa clyton. Recently I reported the successful transformation of a specimen of the tawny emperor butterfly by heparin injection (Sourakov, 2018). That single female individual, in which the dorsal surface of the wings turned almost entirely black, seemed to represent the furthest possible divergence from the normal phenotype.
This result is replicated again here in a male specimen (Figure 4D), with more accurately measured data concerning the injection (Table S1) (Sourakov, 2018). In addition to replicating the previous results, the main purpose of adding this species to the mix was to obtain intermediates between the normal pattern and the most extreme transformations. Variation in resulting phenotypes was achieved by lowering the dose of heparin and using the prepupal stage in addition to the pupal stage. As a result, two intermediate transformations were achieved (Figure 4B, C): the first injected as a prepupa (Figure 4B) and the second as a pupa (Figure 4C). The individual in Figure 4C is not drastically different from the one in Figure 4D, even though the former was injected with three times less heparin than the latter (Table S1) (Sourakov, 2018). The amount of heparin injected into the prepupa (Figure 4B) was between the doses received by the other two transformed individuals.
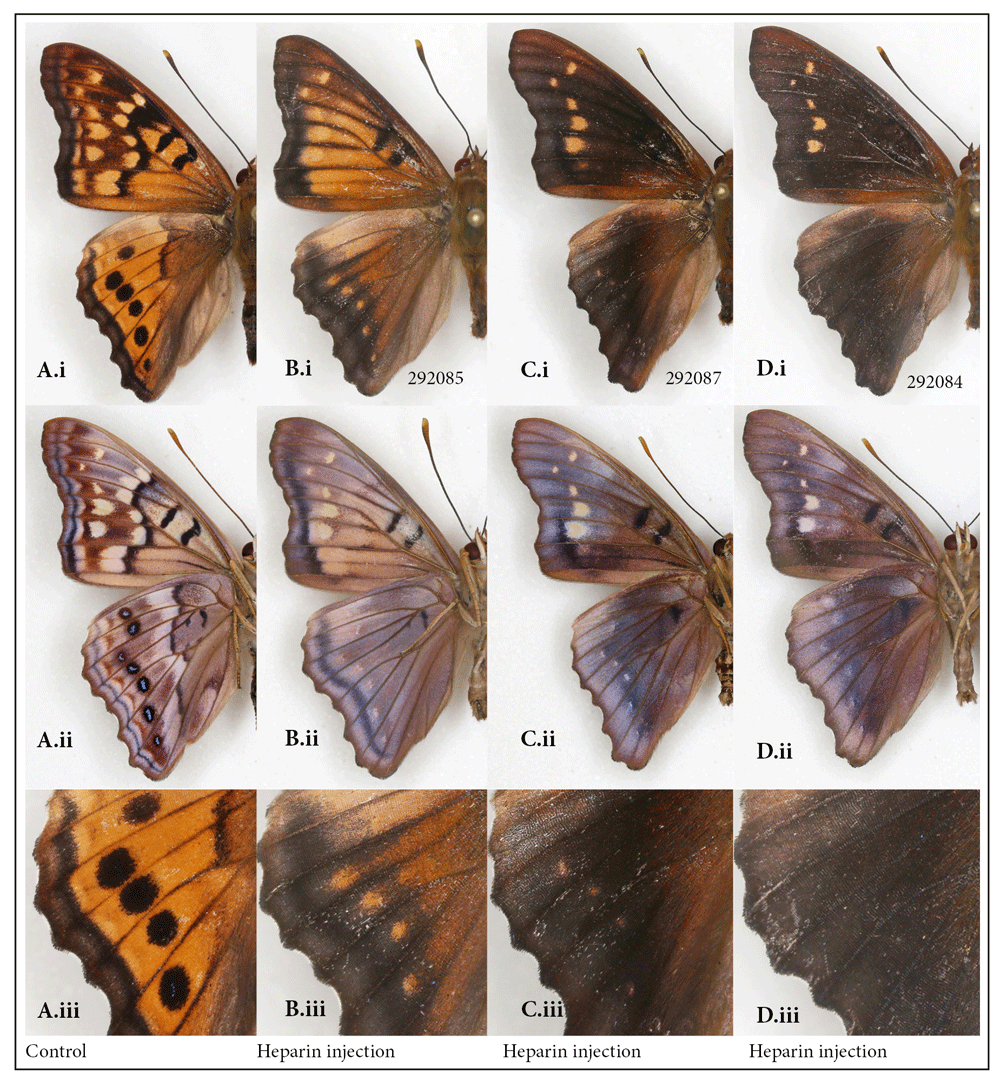
Normal wing pattern (A) vs. heparin-induced wing patterns (B–D) in the males of the tawny emperor, Asterocampa clyton; (i) dorsal, (ii) ventral surfaces, (iii) close-up of border eyespots dorsally. See Table S1 for specimen details.
It is interesting to note that the dorsal hindwing border spots, normally black in this species, rather than disappearing into the black background, as happened in maximally transformed specimens, appeared as orange spots in two of the intermediates (Figure 4(iii)). Marginal eyespots in many Nymphalidae are concentrically organized and serve as models for studies of development, as for example, in Bicyclus anynana, where they have been shown to be positively regulated by Wingless (Wnt) (Özsu et al., 2017). Wnt signaling delimits the boundaries of wing spots, as reviewed by Martin & Courtier-Orgogozo (2017), and is affected by heparin. Figure 4(Aiii–Diii) suggests that, even though the serial border spots of the tawny emperor are not as concentrically organized as in many other nymphalids, they are nevertheless homologous. The marginal bands migrating basally under the influence of heparin are inhibited from invading eyespot centers. One can speculate that these eyespots, like the more concentrically organized ones, are also formed via a signal produced by the few eyespot-organizer cells in the eyespot’s center, as was first described by Nijhout (1980) and recently studied histologically by Iwasaki et al. (2017).
The giant swallowtail, Heraclides cresphontes. While the giant swallowtail is contrastingly patterned species, and one might expect substantial changes to its pattern resulting from heparin injections, this proved to be far from the case. To see the heparin-induced changes one needs to zoom in on the underside of the hindwing, but even then the transformations are barely noticeable. The normally present red spot in the center of the hindwing located on either side of M3 (Figure 5Aiii, Ciii), may become greatly reduced in heparin-injected individuals (Figure 5Biii, Diii). The number of light-colored scales within the ventral hindwing band that give the band iridescence seem to be replaced by black scales, giving these bands a darker appearance (e.g., Figure 5Aii, Bii). These changes are symmetrical, except in the female in Figure 5E, where asymmetry resulted from an asymmetrical defect in vein development, suggesting the influence veins may have on wing pattern formation. Assessing the twenty surviving individuals that were injected with a variety of doses at different stages, it seems that one should target the window around 5 hours after pupation if one wanted to replicate these results.
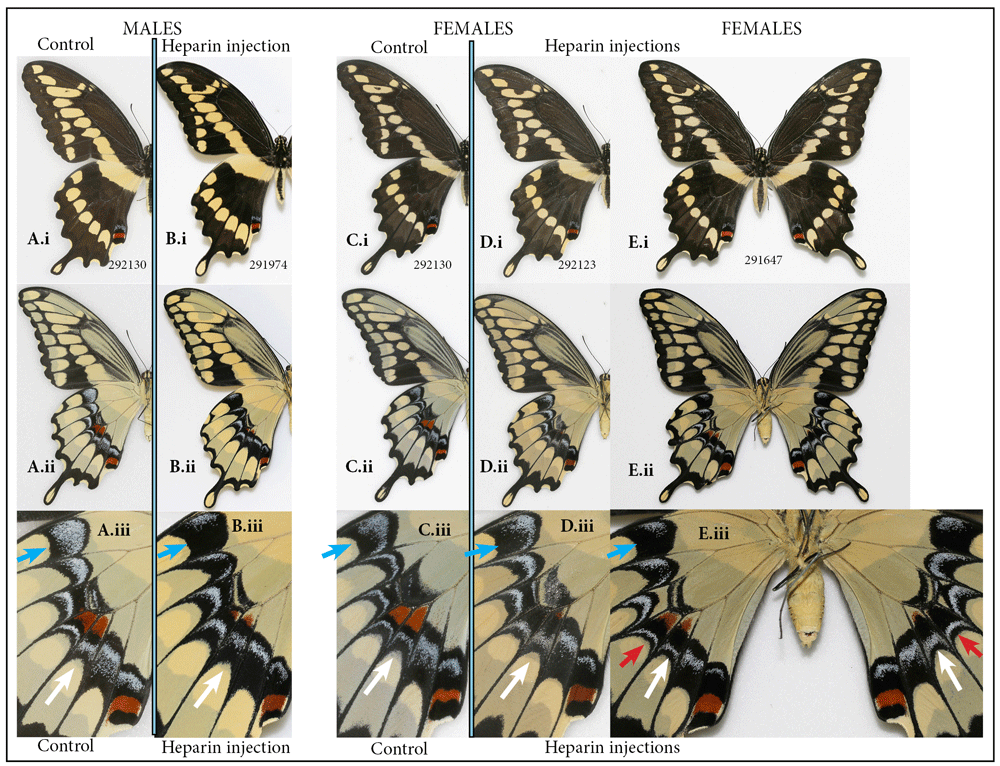
Normal male (A) and female (C) vs. heparin treatment (B, D, E) in the giant swallowtail, Heraclides cresphontes: (i) dorsal, (ii) ventral surfaces, (iii) close-up of hindwing band dorsally. Heparin causes increase in numbers of melanic scales within elements composing ventral hindwing band (blue arrow). Heparin also causes reduction of ventral hindwing central red spot (white arrow). Heparin-induced changes are asymmetrical in an individual with asymmetrically defective wing venation (red arrow). See Table S1 for specimen details.
This species is widespread and can be geographically variable in the characters that demonstrated heparin induced changes (Warren et al., 2013). The present study suggests that both the intraspecific variation in ventral hindwing bands and the variation among species found in the closely-related South American taxa, such as Heraclides paeon, H. homothoas, H. melonius and H. thoas (Tyler et al., 1994 plate 89) would map onto Wnt gene ligand. It also illustrates how wing pattern formation can be compartmentalized in some species, so that variation in gene expression of pattern-mapping genes can affect only some of the compartments, but not others, and it may help to explain the existence of so many species sharing almost identical patterns in the New World.
The spicebush swallowtail, Pterourus troilus. A total of six individuals of the spicebush swallowtail were injected with heparin, and only two survived (both injected as pupae). There was visible transformation of the wing pattern in one of them (injected 5HAP). The central iridescent patch on the dorsal forewing surface that normally extends to, and sometimes beyond, the border of the large turquoise-colored spots (Figure 6Ai, C, D, E) appears to have shifted basally in the transformed individual (Figure 6Bi, Biii). At the same time, the orange spot located on the costal margin of the hindwing was greatly reduced compared to normal (Figure 6Biii, blue arrow). The corresponding changes occurred on the ventral side of the hindwing (Figure 6Bii).
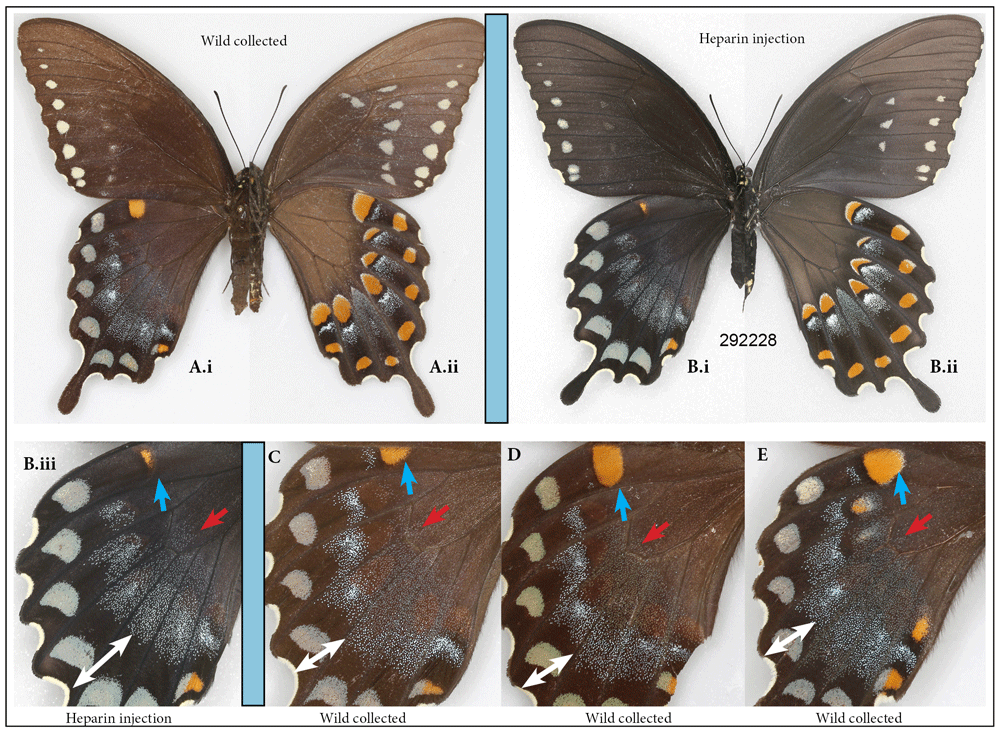
Normal female of the spicebush swallowtail, Pterourus troilus (A) vs. a female that underwent heparin injection as pupa (B): (i) dorsal, (ii) ventral surfaces. Close-up of hindwing dorsally in heparin-injected specimen (B.iii) is contrasted with that of three typical wild-caught specimens (C–E). Heparin causes contraction of dorsal orange spot (blue arrow). Heparin also causes a shift of iridescent hindwing patch basally (white and red arrows). See Table S1 for specimen details.
None of the numerous P. troilus specimens that I examined in the MGCL collection sported this phenotype, giving me confidence, despite limited sample size, that it was heparin-induced. The rare aberrations flava and addenda figured in Warren et al. (2013) might be explained by the results presented here.
The mulberry leaftier moth (Glyphodes sibillalis). This tiny species is relatively large by the standards of micromoths, but it still has a much smaller caterpillar, and hence my expectations of success were low. Nevertheless, among the two surviving experimental individuals, one injected heparin as a prepupa, the other as a pupa, the latter proved to be different not only from the former, but also from the five control specimens. The changes are consistent with heparin-induced changes in other species: the olive-brown hindwing border is wider and darker, and the normally thin and compact submarginal and marginal bands are wider and more diffused on both forewings and hindwings (Figure 7).
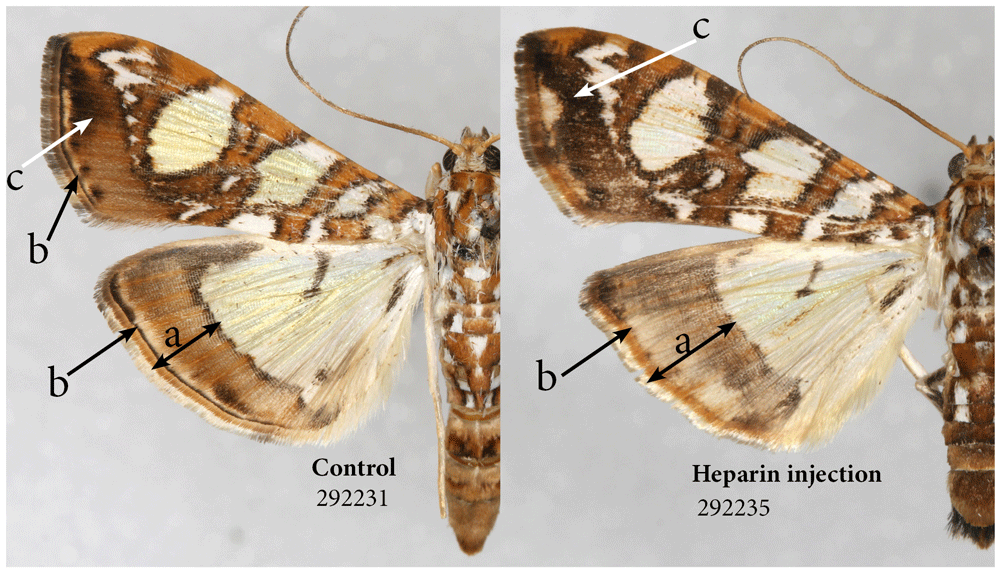
Heparin causes expansion of the border area (a). Heparin also causes expansion of marginal band and diffusion of submarginal band (b) and expansion of some of the melanic territories (c). See Table S1 for specimen details.
Differences in survival by species. It is clear, from the nine species examined in the present study, that heparin treatments are tolerated differently by different species (Table 1). Unlike other species in the present study, very few experimental individuals among the leopard and acrea moths died from heparin injections: 84% of heparin-injected individuals emerged, and even the ones that did not emerge were fully formed inside their pupal cases. Despite the fact that, for the acrea moth, mostly young pupae were injected and, for the leopard moth, almost exclusively prepupae were used, the survival rates were similarly high for both species (Table 1). Compared to some butterfly species, the tiger moths, especially the acrea moth, showed a remarkable resilience to heparin injections, which may be due to their biology, which involves sequestering and synthesizing secondary plant compounds for defense (Singer & Bernays, 2008).
Based on the three swallowtail species used in the present studies, Papilionidae may provide a challenge when it comes to achieving both survival and wing transformation. For instance, one of the species not discussed above, the Zebra swallowtail, Eurytides marcellus, had a zero survival rate, despite attempts to use low concentrations and dosage of heparin at different stages of development and at different conditions. In the giant swallowtail, despite injecting 60 individuals at different stages, visible transformation of wing pattern was difficult to achieve and only a third survived the injection (Table 1). Clearly, more work is needed to optimize my technique; however, considering that these are the first examples from this beautiful family of large, colorful and extremely diverse butterflies with which such manipulations have been conducted, I hope that heparin-induced wing pattern changes described here will contribute to our understanding of the wing pattern evolution as a whole.
Within the Nymphalidae, the survival varied between species and stages. The gulf fritillary was the most successful species, after the tiger moths, in its ability to survive heparin treatment (Table 1), which is perhaps also due to its ability to feed on and sequester toxic compounds from its host plant. However, unlike the tiger moths, the transformation rate was much higher and more predictable. It seems to survive injections and transform equally well as prepupae and pupae. Among the 100 buckeye individuals used in this experiment, the survival of heparin-injected individuals greatly depended on the developmental stage (Table 1). For unknown reasons, success was achieved only with the prepupal stage: 79% (n=19) of the injected as early prepupae and 45% (n=29) of those injected as late prepupae survived. Despite lowering the dose and varying the stage of injection, as well as conducting injections at lower temperatures, achieving the survival of the tawny emperor was more problematic: only 20% of the individuals survived injections, with 4 additional transformed individuals fully formed but unable to emerge from the pupa. It seems that the odds of a successful transformation was higher when injections were made at about 5HAP.
Is wing pattern transformation stage-sensitive? Heparin injections, for the most part do not interfere with pupation, so, almost all the individuals injected as prepupae and subsequently did not survive died in their pupal stage. It is certainly quite likely that, in most species, at least some wing pattern elements are laid down in the prepupal stage, and hence it is important to explore both prepupae and pupae. The logical question becomes: does heparin linger from the time of injection onwards, or does its action correlate strictly with the time of injection? While the similarities in the outcome of late prepupal and pupal injections in the io moth (Sourakov, 2017) may suggest that there is little difference whether a late prepupa or an early pupa is injected, it may be not the case in every Lepidoptera species. There seemed to be a different pattern of transformations that occurred to buckeyes injected as pupae by Serfas & Carroll (2005) from the buckeyes injected as prepupae in the present study: here, the hindwing eyespots remained practically unchanged, throughout the transformation spectrum (except in one individual in which the pattern was completely overhauled, but which was not viable). Serfas & Carroll (2005), on the other hand, were able to gradually decrease the size of the dorsal hindwing eyespot by increasing the dose of heparin.
Among 36 heparin injections in tiger moths, including many where the timing would have been perfect to achieve the transformation in Nymphalidae (ca. 5-8HBP), the only obvious wing pattern transformations occurred in two individuals injected as prepupae. This likely suggests that, at least in these species, the effect is stage-sensitive. My unpublished observations from silk moths also suggest that injecting too early in the prepupal stage does not produce the visible transformations that can be achieved in late prepupal and early pupal stages. Hence, I think that the future researchers pursuing studies of wing pattern of the acrea, leopard and perhaps other tiger moths may want to target the time around 12HBP.
Heparin dosage and stages of metamorphosis. Using the methods as described in this preliminary foray made it extremely difficult to provide an accurate estimate of how much heparin was actually being delivered to the system, because of the variation in dosage and, almost undoubtedly because some heparin may be lost as a result of ‘bleeding’ from the injection site. The pressure in a prepupa may help animal expel any foreign object or liquid attempting to penetrate it.
Another consideration is the changes in weight that a caterpillar undergoes during metamorphosis. My experience with the Io moths, a species in which a normal female can be more than double the weight of a normal male, suggests that the transformation achieved by heparin injections is not only dose- and stage-dependent, but also depends on an animal’s weight. I weighted immature stages of Lepidoptera species that I reared during this study, plus of two additional species of silk moths, the cecropia moth and the imperial moth, that I reared recently. Animals’ weights varied not only with species, but also the same individual’s weight varied greatly depending on the stage of its development (Figure 8). The manner in which weight changed as an animal underwent metamorphosis depended on the family and species it represented. For instance, in butterflies, such as the zebra longwing and gulf fritillary, weight change is minimal, but is more dramatic in the giant swallowtail.
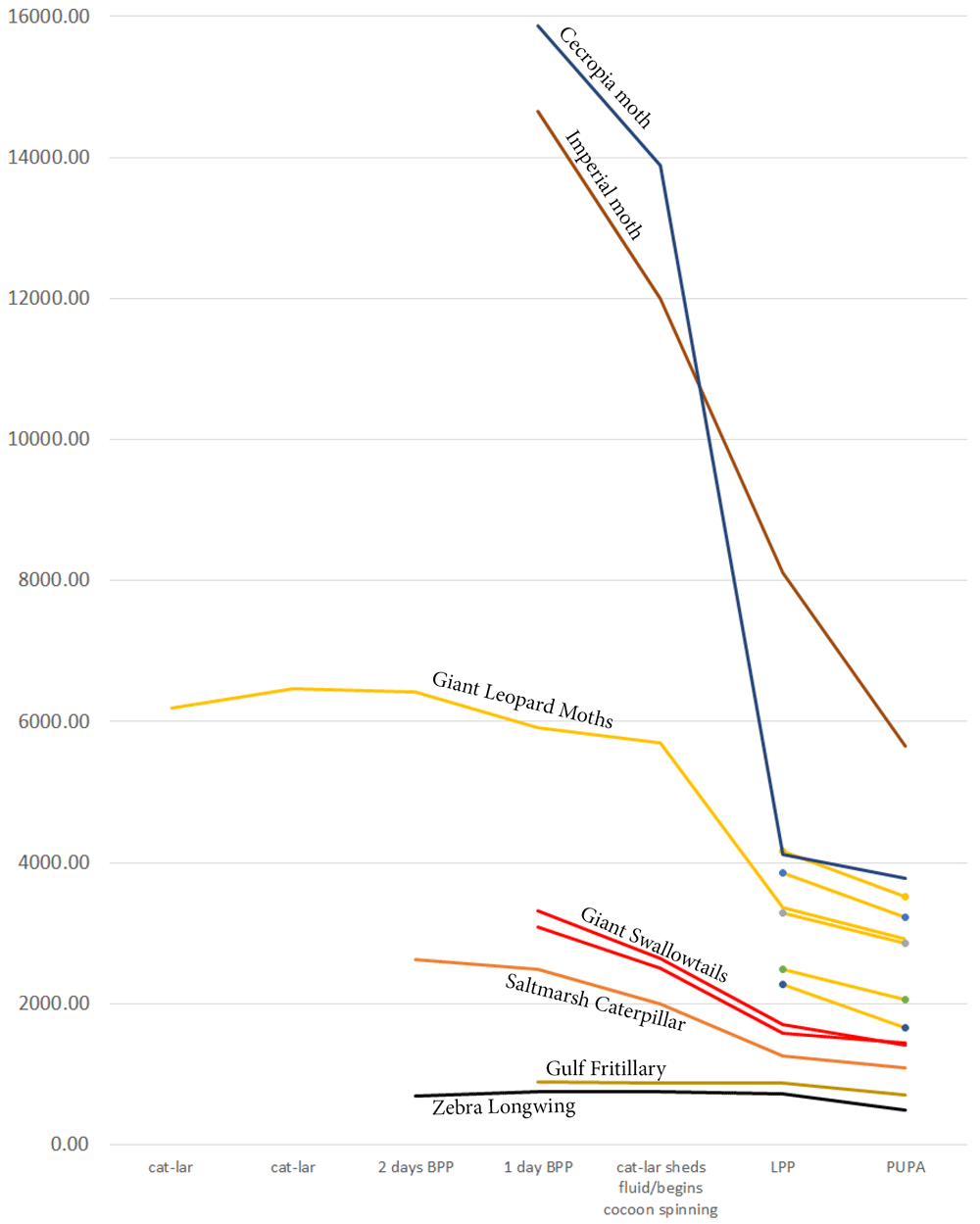
The slope corresponds to species’ biology: moths spinning more silk lose relatively more weight than their counterparts from the same family that spin less silk. Six leopard moth individuals are included to illustrate intraspecific variation. (BPP - before pupation; LPP - late prepupa).
Among moths, even within a single family or subfamily, weight change during metamorphosis may vary depending on a species’ biology, specifically silk production by the caterpillar. For instance, among the wooly bears used in the present study, the caterpillar of the acrea moth (also known as saltmarsh caterpillar) used its own hairs to form its cocoon, thus having much less need for silk, which is mostly used to bind these hairs together. Similar-looking leopard moth caterpillar, on the other hand, spun the cocoon entirely from its own silk, adding droplets of bad-smelling repellent for chemical protection. I suspect this is the reason why a leopard moth caterpillar loses relatively more weight during metamorphosis than a saltmarsh caterpillar (Figure 8). As I already pointed out above, intraspecifically, the weight can also vary substantially, as demonstrated by six leopard moth caterpillars in Figure 8.
Among silk moths, the cecropia moth caterpillar spins a formidable double cocoon, losing ca. 75% of its weight in the process. The imperial moth, on the other hand, though it too belongs to the silk moth family, does not spin a cocoon at all, instead relying for protection on pupating in an underground chamber and on the thick, hard chitin of its pupal case. While its weight loss during the metamorphosis is still significant (ca. 60%), it is noticeably less than in cecropia moth.
Raw data, including raw images and weights of caterpillars and pupa, are available on OSF, DOI: https://doi.org/10.17605/OSF.IO/D2P9H (Sourakov, 2018).
Figure S1. Full spectrum of heparin-induced wing pattern changes in dorsal hindwing of the common buckeye, Junonia coenia (A, B) Control group: (A) males and (B) females injected with H2O as prepupae. (C, D) Experimental group: (C) males and (D) females injected with heparin as prepupae. Wings are arranged left to right demonstrating a gradient in the reduction of the orange band (both groups) and expansion of the marginal bands (experimental group). DOI: https://doi.org/10.17605/OSF.IO/D2P9H (Sourakov, 2018).
Postscriptum-On the term Prepupa, including Figure S2: Morphological recognition of prepupa as a separate stage in Lepidoptera, with further subdivision into early prepupa (epp), prepupa (pp), and pharate pupa (php). (A-B) The common buckeye, Junonia coenia: (A) caterpillar; (B.i) early prepupa; (B.ii) prepupa; (C) The gulf fritillary, Agraulis vanillae: (C.i) early prepupa; (C.ii) prepupa; (C.iii) pharate pupa; (D) the giant swallowtail, Heraclides cresphontes: (D.i) caterpillar; (D.ii) early prepupa; (D.iii) prepupa. DOI: https://doi.org/10.17605/OSF.IO/D2P9H (Sourakov, 2018).
Table S1. Details concerning heparin injections for the specimens illustrated in Figures 1-7. DOI: https://doi.org/10.17605/OSF.IO/D2P9H (Sourakov, 2018).
I thank Matt Standridge for supplying some of the eggs and larvae of the giant swallowtails raised in the course of this study and Julieta Brambila for providing larvae of the mulberry leaftier moth. Alexandra Sourakov kindly proofread the earlier versions of this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Sourakov A: Giving eyespots a shiner: Pharmacologic manipulation of the Io moth wing pattern.F1000Res. 2017; 6: 1319 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Evolution and development of butterfly color patterns, insect developmental genetics, and molecular phylogenetics.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Binari RC, Staveley BE, Johnson WA, Godavarti R, et al.: Genetic evidence that heparin-like glycosaminoglycans are involved in wingless signaling.Development. 1997; 124 (13): 2623-32 PubMed AbstractCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Evolutionary Developmental Biology ; Lepidoptera
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 04 Dec 20 |
read | |
|
Version 2 (revision) 11 Nov 20 |
read | read |
|
Version 1 22 Nov 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)