Keywords
Imunomodulator, Concoction, Aeromonas hydrophila, Pseudomonas fluorescens, Prophylaxis
Imunomodulator, Concoction, Aeromonas hydrophila, Pseudomonas fluorescens, Prophylaxis
Tilapia (Oreochromis niloticus) is one of the most widely cultivated fish species in Indonesia. Tilapia is a freshwater fish that can be easily cultivated1. According to Pridgeon2 and Harikrishnan et al.3, freshwater fish culture is inseparable from bacterial infections which are caused by motile Aeromonas septicaemia, furunculosis, edwardsiellosis and Aeromonas hydrophila. Further, Aeromonas species have been identified as major causative bacteria and a serious pathogen in fish4,5. In Indonesia, particularly East Kalimantan, infection of A. hydrophila and Pseudomonas fluorescens in fish results in high mortality rates of up to 60–80%. In fish, both of these bacteria cause stresses, exophthalmia, ulcers, and watery-looking organs, particularly gallbladder rupture6–8. In addition, combined bacterial infection in fish is also common, such as infections found in tilapia caused by Salmonella agalactiae and A. hydrophila9,10.
To reduce high mortalities of cultured fish, aquaculturists and researchers use antibiotics to prevent and treat infection. Nevertheless, due to concerns for maintaining eco-friendly environments, the application of antibiotics should be avoided, because they may enhance antibiotic-resistant pathogens, increase the accumulation of drugs in fish tissue and trigger immunosuppression11. Methods of controlling these infections should be developed as soon as possible because the pathogen disease type has significantly increased12, while the type of pathogen that leads to edema in the cultivation area still cannot be overcome. One of the effective and safe methods for disease control in aquaculture is by improving the defence system of the fish through the provision of natural immunostimulants13, through the use of several plant extracts.
Various plant extracts, such as Indian almond leaves (Terminalia catappa), oats (Avena sativa), oyster mushroom (Pleurotus ostreatus), nettle (Urtica dioica), sea grass (Cymodocea serrulata) and beetroot (Beta vulgaris) have been used as alternatives to antibiotics5,14–16. Plant extracts also contain levamisole13 and saponin17 which can enhance the work of nonspecific immune systems and increase the activation of phagocytosis14. Further, the plant extracts of Boesenbergia pandurata (BP) and Zingiber zerumbet (ZZ) from East Kalimantan have in vitro and in vivo antibacterial activity against A. hydrophila bacteria, while Solanum ferox (SF) has been found to be an antibacterial agent for P. fluorescens bacteria. Similarly, for the prevention and treatment of bacterial infections in tilapia, BP and ZZ are also effective for treating A. hydrophila and P. fluorescens infection8,18.
The incorporation of some extracts for the prevention and treatment of bacterial infections is likely to increase the effectiveness because some materials can work synergistically, so that the infection of both bacteria in the fish body can be controlled optimally. However, research regarding the combination of plant extracts to treat and prevent bacterial infection is limited. This study therefore aims to determine the effectiveness of the combination of three extracts (BP, ZZ and SF) to prevent and treat bacterial infections of A. hydrophila and P. fluorescens in tilapia.
In total, 450 Tilapias (Initial weight 15 ± 2 g, age ±2.5 months, random sex) were obtained from Teluk Dalam Village in Tenggarong Seberang, Kutai Kartanegara, Indonesia. The fish were randomly distributed and assigned into five aquariums in triplicate, representing four treatments and one control. The fish were kept in the laboratory for two weeks for acclimatization in the aquarium (60×40×30 cm). Each aquarium was filled with 60 l of freshwater and the water was changed by as much as 50% every 2 days to remove remaining faeces and inedible feed. The average temperature of the water was 27°C. The feed given in the acclimation phase was a commercial feed (PT Rama Jaya Mahakam, Kutai Kartanegara East Kalimantan-Supplier) at a rate of 5% of the body weight of the fish per day. The bacteria used for the challenge test were A. hydrophila (EA-01) and P. fluorescens (EP-01), which was provided from the Aquatic Microbiology Laboratory, Faculty of Fisheries and Marine Sciences, Mulawarman University, Indonesia. To bring about bacterial challenge, a combination of bacteria at density of 105 CFU ml-1 of each bacteria was used. Each fish was injected intramuscularly with 0.1 ml of the suspension of the bacteria.
The plant materials, BP, SF and ZZ, were collected from a traditional market in Samarinda City, East Kalimantan, Indonesia. The plants were cleaned, cut and dried at 40°C for 48 hours in the oven, finely powdered and stored at -4°C for the further extraction stage. Ethanol solution (95%) was used to extract the plant materials, following a method described by Limsuwan & Voravuthikunchai19.
This treatment and prevention trials were carried out for 28 days. The treatment experiments were conducted with five combination treatments with the following stages: tilapia (average initial weight 15 ± 2 g, n = 30 fish per group, random sex) were injected intramuscularly (0.1 ml) with a mixture of A. hydrophila and P. fluorescens bacteria, each bacteria at density105 CFU ml-1. At day 7 after injection, the fish were fed with feed combined with extract as follows (ml per kg feed): P1, 60 ml SF extract/kg feed with 40 ml ZZ extract/kg feed (SF60/ZZ40); P2, SF50/ZZ50; P3, BP90/SF10; P4, BP50/SF50; and P5, fed with no additional extract (control). All fish were fed twice a day ad satiation. The remaining feed was siphoned out before the next feeding.
Meanwhile, the prevention trial was performed by providing the same feeding combination and procedure for 6 days prior to intramuscular injection of the fish with 0.1 ml of mixed bacteria at day 7. After injection, feeding combination was continued until the 4th week. Haematological and immunological parameters were measured every week after the injection with bacteria until week 4.
At days 14, 21 and 28 following bacterial challenge, haematological profiles of fish (n=3 per treatment group) were observed. Fish were anesthetized using 50 mg l-1 MS 222 (Sigma Aldrich, USA) per dm3 water. The fish blood was taken through the caudal vein, using a 1 ml syringe rinsed with 10% trisodium citrate anticoagulant (fish were kept alive after blood withdrawal). Total red blood cells (RBC) (106 per mm3) and white blood cells (WBC) (103 per mm3) were determined manually using an improved Neubauer counting chamber. The number of WBC was calculated using the method of Blaxhall and Daisley20. Haemoglobin (Hb) was measured spectrophotometrically at 540 nm using the cyanmethemoglobin method17. The haematocrit (Htc %) was counted using the microcentrifuge and heparinized was used as a standard solution. Meanwhile, phagocytic activity was determined using a modification of previous methods20,21.
To obtain serum, the fish blood was taken from the caudal veins and collected in an Eppendorf tube and centrifuged at 3,000 rpm for 3 minutes. Serum was then incubated at 44°C for 20 min to activate the complement22. Serum was stored in the refrigerator at 4°C for the next antibody titre observation. Measurements of antibody titres were performed using 25 μl PBS and inserted into microplate at holes 1 to 12, with the serum being inserted into hole 1 (25 μl) and then diluted into 11 holes. A total of 25 µl of bacteria (A. hydrophila and P. fluorescens) were inserted into holes 1 to 12, the mixture homogenized, and stored for 2 hours in an incubator at 37°C, followed by storing at 4°C overnight in a refrigerator. For analysis, observing the antibody titre was carried out, indicated by the agglutination reaction in the last hole.
Respiratory burst activity test was performed using nitro blue tetrazolium (NBT) reagent, using the method outlined by Secombes and Olivier23. Meanwhile, lysozyme activity was performed using a microtiter plate ELISA reader at wavelength of 520 nm, following the method described by Soltani and Pourgholam24.
Both A. hydrophila and P. fluorescens (the pathogenic bacteria) were used for challenge testing (n = 10 fish per aquarium, in triplicates per group). The survival rate (SR) and relative percent survival (RPS) of the fish were recorded on a daily basis for 4 weeks25.
Results are expressed as means ± standard error (SE) and the data were analysed using SPSS version 22 (SPSS, Inc., USA). The data of WBC, RBC, haematocrit, Hb, TPC, phagocytic index, respiratory burst and lysozyme activity were subjected to ANOVA, followed by Duncan’s post hoc test to evaluate significant differences among the groups of treatments. The percentage of fish survival were arcsine-transformed. All tests were significant at P < 0.05.
The present results revealed that the total WBC count of tilapia in the treatment and prevention trials were significantly increased (P<0.05) from weeks 2–4 post-administration with combined extracts. The highest increase of WBC was found in tilapia fed with SF50/ZZ50. Similarly, total RBC and haematocrit of tilapia fed SF50/ZZ50 in the treatment trial showed a significant increase after week 2, while tilapia fed SF60/ZZ40 in the prevention trial led to a positively enhanced result from weeks 2–4. Further, haemoglobin of fish both in treatment and prevention trials were not affected by any various combination of extracts (Table 1).
| Variables | Trials | Groups | Weeks | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| WBC (104 cell/mm3) | Treatment | SF60/ZZ40 | 1.53±0.1a | 1.68±0.1a | 1.62±0.1a | 2.07±0.2b |
| SF50/ZZ50 | 2.55±0.02ab | 3.60±0.1b | 3.90±0.2b | 8.85±0.2c | ||
| BP90/SF10 | 1.87±0.2ac | 1.88±0.5a | 2.00±0.1a | 2.10±0.1a | ||
| BP50/SF50 | 1.98±0.5ac | 1.89±0.5a | 2.02±0.2a | 2.20±0.1b | ||
| No extract | 1.35±0.3a | 1.35±0.2a | 1.32±0.2a | 1.34±0.1a | ||
| Prevention | SF 60/ZZ 40 | 1.5±0.5a | 1.8±0.15a | 1.7±0.2a | 2.4±0.5b | |
| SF 50/ZZ 50 | 2.8±0.3a | 3.9±0.2b | 4.0±0.1b | 7.9±0.2c | ||
| BP 90/SF 10 | 1.8±0.15a | 2.0±0.2a | 2.4±0.1b | 2.4±0.3b | ||
| BP 50/SF 50 | 2.0±0.25a | 2.0±0.3a | 2.2±0.2a | 2.5±0.1b | ||
| No extract | 1.4±0.1a | 1.4±0.5a | 1.3±0.3a | 1.3±0.1a | ||
| RBC (106cell/mm3) | Treatment | SF 60/ZZ 40 | 6.9±0.1a | 5.4±0.3a | 4.2±0.2b | 6.0±0.1b |
| SF 50/ZZ 50 | 6.9±0.1a | 7.7±0.2b | 8.8±0.1c | 8.9±0.2c | ||
| BP 90/SF 10 | 5.1±0.2a | 6.3±0.1b | 7.2±0.1c | 5.4±0.2a | ||
| BP 50/SF 50 | 5.5±0.11a | 6.8±0.2a | 7.0±0.1b | 7.7±0.1b | ||
| No extract | 2.4±0.3a | 2.7±0.1a | 2.7±0.2a | 2.4±0.1a | ||
| Prevention | SF 60/ZZ 40 | 6.9±0.15a | 6.4±0.25a | 5.2±0.2b | 6.0±0.5b | |
| SF 50/ZZ 50 | 6.9±0.2a | 7.0±0.3a | 7.2±0.1a | 7.0±0.2a | ||
| BP 90/SF 10 | 5.1±0.1a | 6.2±0.1a | 5.5±0.1a | 5.4±0.2a | ||
| BP 50/SF 50 | 5.5±0.1a | 5.6±0.1a | 7.0±0.1a | 7.0±0.1a | ||
| No extract | 2.4±0.2a | 2.7±0.2a | 2.7±0.2a | 2.4±0.1a | ||
| Haematocrit (%) | Treatment | SF 60/ZZ 40 | 27±0.1a | 27±0.1a | 30±0.1a | 33±0.1b |
| SF 50/ZZ 50 | 22,5±0.5a | 27±0.2b | 33±0.1c | 36±0.2c | ||
| BP 90/SF 10 | 25,5±0.5a | 27±0.2a | 30±0.1a | 31±0.2a | ||
| BP 50/SF 50 | 27±0.2a | 27±0.2a | 27±0.2a | 30±0.2a | ||
| No extract | 18±0.2a | 15±0.3a | 18±0.1a | 15±0.2a | ||
| Prevention | SF 60/ZZ 40 | 30±0.5a | 27±0.1a | 27±0.1a | 28±0.1a | |
| SF 50/ZZ 50 | 22,5±0.1a | 27±0.2a | 33±0.2a | 33±0.1a | ||
| BP 90/SF 10 | 25,5±0.1a | 27±0.1a | 30±0.2a | 31,5±0.1a | ||
| BP 50/SF 50 | 27±0.2a | 27±0.1a | 27±0.1a | 30±0.2a | ||
| No extract | 18±0.1a | 15±0.2a | 18±0.1a | 15±0.1a | ||
| Haemoglobin (g dl-1) | Treatment | SF 60/ZZ 40 | 8±0.1a | 10±0.3a | 10±0.1a | 10±0.1a |
| SF 50/ZZ 50 | 10±0.2a | 10±0.3a | 10±0.2a | 10±0.1a | ||
| BP 90/SF 10 | 8±0.11a | 8±0.2a | 10±0.2a | 10±0.1a | ||
| BP 50/SF 50 | 8±0.1a | 8±0.2a | 8±0.2a | 10±0.1a | ||
| No extract | 6.3±0.5a | 8±0.1a | 6±0.2a | 6±0.2a | ||
| Prevention | SF 60/ZZ 40 | 8±0.2a | 10±0.1a | 8±0.2a | 8±0.2a | |
| SF 50/ZZ 50 | 10±0.2a | 10±0.1a | 8±0.2a | 10±0.1a | ||
| BP 90/SF 10 | 8±0.2a | 10±0.1a | 8±0.1a | 8±0.1a | ||
| BP 50/SF 50 | 8±0.2a | 8±0.1a | 8±0.1a | 8±0.2a | ||
| No extract | 6.3±0.2a | 8±0.1a | 6±0.1a | 4±0.1a | ||
Data shown as mean±standard deviation. Different superscript letters (a,b,c) in the same column in each variable and each treatment or prevention trial showed significantly different at P<0.05. WBC, white blood cells; RBC, red blood cells; BP, Boesenbergia pandurate; SF, Solanum ferox; ZZ, Zingiber zerumbet; SF60, 60 ml SF extract/kg feed.
All combination extracts fed to fish in the treatment (Figure 1) and prevention (Figure 2) trials increased the phagocytic index. The phagocytic index of fish fed SF50/ZZ50 in the diet, in both in treatment and prevention trials, were significantly higher than control and increased from the 2nd to 4th week of the post-challenge test.
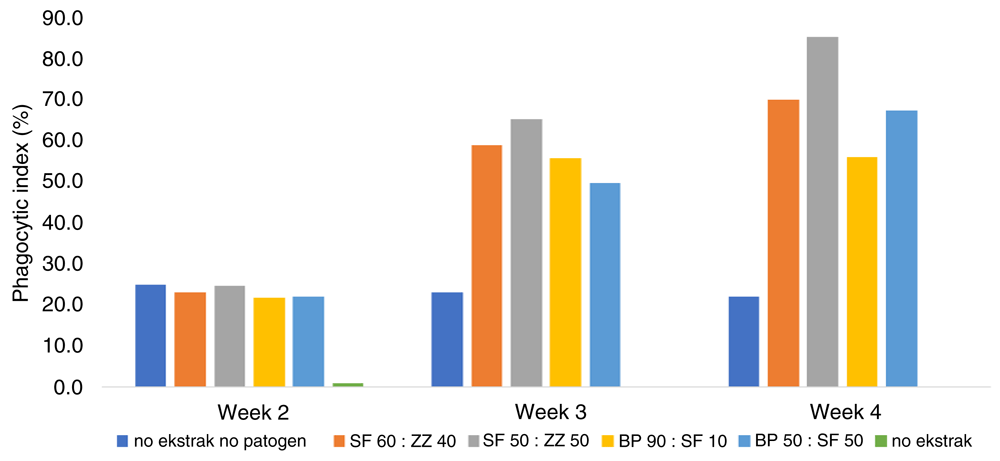
BP, Boesenbergia pandurate; SF, Solanum ferox; ZZ, Zingiber zerumbet.
The respiratory burst activity of infected fish fed with combination extract increased from week 2 to week 4 in the treatment trial (Figure 3). In addition, SF50/ZZ50 (ml per kg feed) combination extract resulted in a significantly different respiratory burst to other combinations of extracts and the control. Meanwhile, in the prevention test, infected fish fed SF50: ZZ50 combination extract in week 4 were significantly higher than control and other combinations of extracts (P<0.05) (Figure 4).
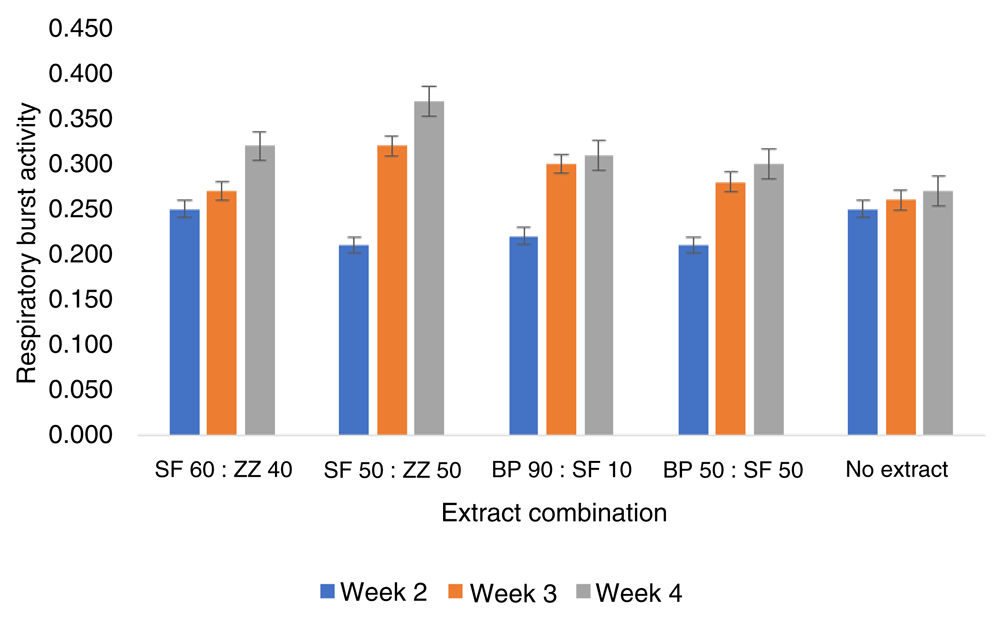
BP, Boesenbergia pandurate; SF, Solanum ferox; ZZ, Zingiber zerumbet.
This study revealed that lysozyme activity of infected tilapia fed SF60/ZZ40, BP90/SF10 and BP50/SF50 combinations of extract did not show a significant increase (P<0.05) at weeks 2 and 4 in the treatment test. However, starting from weeks 2–4, the addition of SF50/ZZ50 combination extract in the diet of fish resulted in significantly better lysozyme activity (Figure 5). Meanwhile, in the prevention test at weeks 2 and 4, the lysozyme activity of tilapia fed SF50/ZZ50 was significantly higher (P<0.05) (Figure 6) than in other combinations.
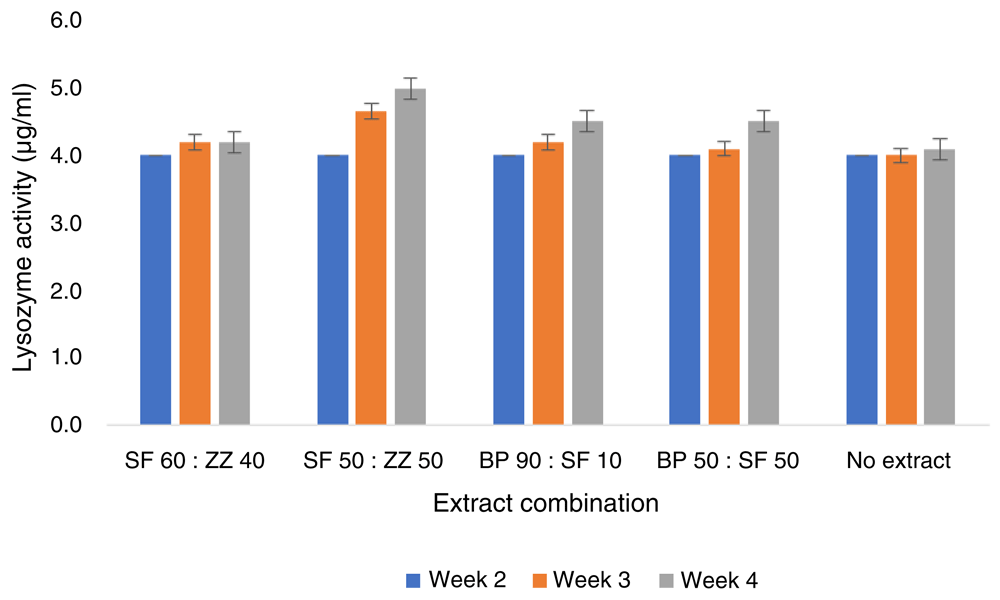
BP, Boesenbergia pandurate; SF, Solanum ferox; ZZ, Zingiber zerumbet.
The overall combination of extracts administered to treat and prevent infection by A. hydrophila and P. fluorescens may decrease the number of bacteria in the fish body until the 4th week of observation (Table 2). The bacterial density, in both the treatment and prevention trials was lower than in the control. Total bacteria of A. hydrophila and P. fluorescens in tilapia fish fed combination extract in the treatment trial decreased from weeks 2–4. Further, the lowest bacterial density in tilapia was obtained from the fish fed SF 50/ZZ 50 combination extracts in their diet, which was also significantly different (P<0.05) compared to the control.
| Trials | Groups | Week | |
|---|---|---|---|
| 2 | 4 | ||
| Treatment | SF 60/ZZ 40 | 17.4±5a | 10.86±10c |
| SF 50/ZZ 50 | 22.4±15a | 3.05±10d | |
| BP 90/SF 10 | 55±10b | 16±15c | |
| BP 50/SF 50 | 47±10b | 4.82±10d | |
| No extract | 42.85±15b | 30.9±5b | |
| Prevention | SF 60/ZZ 40 | 78±15a | 56±15a |
| SF 50/ZZ 50 | 100±5b | 32±10d | |
| BP 90/SF 10 | 65±10a | 46±5d | |
| BP 50/SF 50 | 147±11c | 85±10a | |
| No extract | 157±11c | 110±10c | |
The administration of extract with different combinations on tilapia injected with A. hydrophila and P. fluorescens bacteria increased the SR and RPS when compared to those not given the extracts (Table 3 and Table 4). The percentage of survival of tilapia in treatment and prevention trials with combination extracts of SF 50: ZZ 50 had the highest SR compared to the other combinations of extract.
| Trials | Groups | Week | |
|---|---|---|---|
| 2 | 4 | ||
| Treatment | SF 60/ZZ 40 | 78.00a | 78.00a |
| SF 50/ZZ 50 | 90.00a | 86.00a | |
| BP 90/SF 10 | 83,30a | 77.00a | |
| BP 50/SF 50 | 86.00a | 85.00a | |
| No extract | 45.00b | 33.30b | |
| Prevention | SF 60/ZZ 40 | 85.00a | 75.00a |
| SF 50/ZZ 50 | 100.00b | 90.00b | |
| BP 90/SF 10 | 90.00b | 80.00a | |
| BP 50/SF 50 | 85.00a | 75.00a | |
| No extract | 45.00c | 29.00c | |
| Trials | Groups | Week | |
|---|---|---|---|
| 2 | 4 | ||
| Treatment | SF 60/ZZ 40 | 60a | 67a |
| SF 50/ZZ 50 | 82b | 79b | |
| BP 90/SF 10 | 70a | 66a | |
| BP 50/SF 50 | 75b | 78b | |
| No extract | |||
| Prevention | SF 60/ZZ 40 | 73a | 65a |
| SF 50/ZZ 50 | 100b | 86b | |
| BP 90/SF 10 | 82a | 72a | |
| BP 50/SF 50 | 73a | 65a | |
| No extract | |||
The number of infectious diseases caused by pathogenic bacteria such as A. hydrophila have become a pivotal concern in fish culture, causing high economic losses owing to high mortality rates5. The use of plant-based extracts as immunodulators has been applied to increase survival and immune system of fish to prevent or cure bacterial pathogen. Several plant extracts that contain active phytochemicals have been found and used as supplements in the feed of fish25–28.
The current study found that the WBC of tilapia infected by both bacteria in the prevention and treatment trials increased significantly (P<0.05), while the RBC of tilapia infected by both bacteria in the prevention and treatment trials decreased significantly (P<0.05). This result is similar to those of a previous study, which stated that the WBC increased in order to tackle the infection, while the RBC was decreased in tilapia infected with Streptococcus agalactiae bacteria29, S. iniae10, A. hydrophila and Pseudomonas sp.7. In contrast, tilapia fed with a combination of extracts SF60/ZZ40 showed a similar RBC value both in treatment and prevention trials. In addition, tilapia fed SF50/ZZ50 in treatment trial resulted the highest RBC at the end of the trial. The Hb and Htc values were unchanged during the first week of all treatments including control; the decrease in Htc and Hb values occurred in controls without extract from weeks 2–4 post-infection in the prevention and treatment trials. This result indicated that the combined administration of the extracts was capable of improving the performance of the fish immune system by producing more WBC, thus making the fish more able to suppress the growth of bacteria in the body.
RBC, WBC, Hb and Htc can be used as an indicator of the blood profile in fish with respect to the innate immune defence and regulation of immunological function30. WBC are particularly responsible for providing protection or resistance to disorders caused by infectious pathogens and non-infectious factors (nutrition, temperature and handling)31. Total value of WBC also describes the health status and immune system of the fish. In addition to haematological statues, the Hb content decreases due to RBC swelling and poor Hb mobilization of the spleen and other haematopoesis organs32.
Besides blood profiles, the phagocytic index, respiratory burst and lysozyme activity are good indicators for immunological status of fish during infection periods. The present results revealed that infected fish treated with a compound extract of SF50/ZZ50 showed the highest IP and increased from weeks 2–4 post-injection. These results are in line with the results of a previous study, which found that fish treated with immunostimulants usually show enhanced phagocytic cell activities33. Fish have several types of phagocytic leukocytes, which are part of WBC, in the peritoneal cavity, and various tissues. The phagocytic activity is also associated with the production of oxygen free radicals by using respiratory bursts, which are important events in bactericidal pathways in fish34,35. In addition, Secombes and Olivier23 revealed that the release of superoxide anions, hydrogen peroxide and hypochlorous acid into the phagosome and extracellular space during the respiratory burst can be considered the pivotal mechanisms involved in the bactericidal activity of macrophages.
Total lysozyme level is a tool to measure the humoral component of the non-specific defence mechanism (innate immunity), which can be used to detect infections or injections of foreign material, including bacteria36–38. The present findings determined that tilapia fed SF 50: ZZ 50 had significantly higher (P<0.05) lysozyme activity. This finding is in line with past research, stating that the lysozyme activity of Jian carp (Cyprinus carpio var. Jian)39 and large yellow croaker, Pseudosciaena crocea40 were increased after being fed with traditional Chinese medicine formulated from Astragalus root (Radix astragalin seu Heydsari) and Chinese Angelica root (R. angelicae Sinenesis).
A combination of plant extracts was found to affect the health status of tilapia when compared with control. A combination of extracts of SF and ZZ (50:50) provides the optimum protection against bacterial infections of A. hydrophila and P. fluorescens in both prevention and treatment assays.
Raw data for Tables and Figures can be accessed on OSF, DOI: https://doi.org/10.17605/OSF.IO/A42JB41.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
This research is supported by the Ministry of Research and Technology of the Republic of Indonesia for the support of research funds provided through the National Strategic Research Institutions Fiscal Year 2018, contract No. 121/UN17.41/KL/2018.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The research team would like to thank the Department of Aquaculture, Faculty of Fisheries and Marine Sciences Mulawarman University, East Kalimantan, for the support of facility and equipment during the research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Yudiati E, Isnansetyo A, Murwantoko, Ayuningtyas, et al.: Innate immune-stimulating and immune genes up-regulating activities of three types of alginate from Sargassum siliquosum in Pacific white shrimp, Litopenaeus vannamei.Fish Shellfish Immunol. 2016; 54: 46-53 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology, microbiology, fish diseases, natural products
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Zahran E, Abd El-Gawad EA, Risha E: Dietary Withania sominefera root confers protective and immunotherapeutic effects against Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus).Fish Shellfish Immunol. 2018; 80: 641-650 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical biochemistry, Lipid chemistry, Bioactive compounds characterization
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 13 Feb 19 |
read | read | read |
|
Version 1 26 Nov 18 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Thank you for your valuable comments. We will improve our article.
Thank you for your valuable comments. We will improve our article.
Thank you for your valuable comments. We will improve our article.