Keywords
Culture media, chicken feather, keratin, hydrolysate, bacteria, yeasts, growth rate, protein source
Culture media, chicken feather, keratin, hydrolysate, bacteria, yeasts, growth rate, protein source
Microbial culture medium is composed of different nutrients required by organisms for growth. The nutrient requirements of microorganisms differ from one to another as there are many types of microorganisms. Generally, the microbial growth composition includes carbon and energy sources, protein hydrolysates, otherwise known as peptones, extracts, buffers and sometimes gelling agents. Microorganisms will only be able to grow if they are provided with the appropriate nutrients for growth. Apart from carbon, microbes require a source of nitrogen. Amino acids, urea, ammonia and other compound may serve as the nitrogen source. Some organisms also possess the ability to metabolize peptides and more complex proteins (Sandle, 2016).
The protein source for microbial culture is derived from peptone, which is a good source of amino acids, peptides and proteins in growth medium. It is an excellent source of nitrogen. However, it is also a very expensive constituent of the microbial culture medium (Taskin & Kubanoglu, 2011). Different natural products, such as milk, animal tissues and plants, are being exploited for obtaining peptone in order to reduce the costs of production of the growth medium (Jayathilakan et al., 2012; Ben Rebah & Miled, 2013).
It is estimated that about 20 million tons of chicken feathers are generated weekly worldwide and are considered menaces in terms of solid waste pollution (Egelyng et al., 2018; Tesfaye et al., 2018). In a bid to dispose of the large amounts waste chicken feathers generated worldwide, methods such as landfill and burning have been used, which have taken a toll on the environment (Sharma et al., 2017).
To reduce the burden of waste generated by disposed chicken feathers, a number of processes and operations involving the application of chicken feathers have been reported. Chicken feathers have been employed in the production of animal feed, textile production and paper production amongst many others (Chinta et al., 2013; Moritz & Latshaw, 2001; Tesfaye et al., 2017).
Chicken feathers contain more than 90% protein (keratin), 1% lipids and 8% water (Lasekan et al., 2013). Keratin proteins are grouped into the alpha and beta keratins. Chicken feathers and feathers from most birds are composed majorly of the beta-keratin. Keratin contains all 20 amino acids linked together by peptide bonds, which include covalent disulphide bonds, ionic bonds, hydrogen bonds and hydrophobic bonds (Greenwold et al., 2014). Keratins are bonded by a number of these bonds which make them naturally insoluble. These bonds require that they be broken in other to obtain the chicken feathers in usable forms for microorganisms. By hydrolysis, the bonds are broken, a soluble product is formed (hydrolysate) and the peptone can be obtained from the chicken feather keratin (Ayutthaya & Wootthikanokkhan, 2013). The conversion of such large amounts of chicken feathers into hydrolyzed forms for the manufacture of microbial culture medium can be used as a measure of solving this problem. The chicken feather keratin can then be incorporated into the production of microbial culture medium. This study was therefore targeted at utilizing the keratin in the waste chicken feathers as a cheaper alternative to peptone and also a nitrogen source for microbial growth.
Chicken feathers were collected from the poultry house in Landmark University Commercial Farm in Omu-Aran, Kwara State, Nigeria. The feathers were first washed with water and laundry detergent before disinfecting with 5% hypochloride solution. Following disinfection, the feathers were sun-dried for one week and stored in baskets until when needed.
For hydrolysis, approximately 400 g of dried chicken feathers were placed in 10-l plastic container, after which 2000 ml 1 M NaOH was added. The feather-NaOH mixture was stirred vigorously and left to stand for 10 h. After hydrolysis, the mixture was filtered with a clean, dry muslin cloth and the unhydrolyzed fraction estimated. The quantification of the unhydrolyzed fraction was carried out after drying in an oven to constant weight and then weighed to ascertain the degree of hydrolysis.
Feather keratin was precipitated separately from the hydrolyzed feather solution, using 1 M solutions of the following acids: HCl, H2SO4, C2HCl3O2 (TCA) and HNO3. This was carried out for 10 min at 25°C.
The precipitated chicken feather hydrolysate was separated from the solution by filtration. The chicken feather hydrolysate of the respective acids was air-dried and quantified. The chicken feather hydrolysate of the respective acids (henceforth referred to as feather keratin or hydrolysate) were ground into fine powders using a laboratory blender.
Fresh cattle blood was obtained from the abattoir of Landmark University, Omu-Aran (Nigeria) Teaching and Research Farm. The blood was immediately heated at 60°CC for 30 min to coagulate. The coagulated blood was then placed in aluminium foil and oven-dried at 50°C for 8 h. The dried coagulated blood was milled into powder, using a mechanical grinder. The powdered blood, referred to as blood meal (BM) was kept in air-tight container until required.
For this study, different compositions of feather keratin, peptone (Oxoid, UK) and BM were used in the medium formulations. Five different combinations were used to make the respective growth medium, as follows. Combination A: 60% keratin, 40% peptone, 0% BM; Combination B: 60% keratin, 20% peptone, 20% BM; Combination C: 60% keratin, 0% peptone, 40% BM; Combination D: 100% peptone; Combination E: 100% BM.
The respective components were weighed separately and dissolved in aliquot quantities of distilled water before combining all the components together to make a final medium concentration of 5 g/l. Each medium, as composed, was dispensed in conical flask and sterilized in an autoclave (121°C for 15 min) at 15 psi.
A total of eight microbial species, consisting of five bacteria and three yeast species were used for the study. The bacteria species were Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Staphylococcus aureus and Pseudomonas aeruginosa. The yeasts were Candida carpophila, Candida tropicalis and Pichia kundriavzevii.
Prior to use, stock microbial cultures of the isolates were first streaked on agar plates to ascertain their purity before subculturing into nutrient broth. Equal volumes of broth cultures of the pure isolates (0.5 ml) were used for inoculation. Bacteria and fungi were incubated at 24±2°C.
For growth studies, the flasks containing the sterile medium were inoculated with the test microbial species. Following inoculation and every 2 h, for a 10 h duration, aliquot samples were withdrawn from each flask to monitor growth by measuring absorbance using a spectrophotometer at a wavelength of 750 nm, using sterile nutrient broth to normalize. Each experiment was carried out in duplicate.
Data analysis was carried out using the SPSS statistical software, version 13.0. Comparison of means was determined using the one-way ANOVA test at a significance level of P<0.05. For the least-significant-difference post hoc multiple comparison test was used. All experimental setups were in duplicates.
Raw absorbance values for each microbe are available on figshare (Akpor et al., 2018).
As shown in Figure 1, the growth pattern of Candida carpophila in all the acid hydrolysate medium showed consistent increase with time. The highest growth was observed in the medium with 100% peptone. This trend was irrespective of the acid hydrolysate used. Besides, the 100% peptone medium, the ranking of the growth of the organism from the highest to the least in the other medium compositions varied for each acid feather hydrolysate. In the TCA feather hydrolysate medium, after 10 hours, the organism recorded the highest growth in the 100% peptone medium, closely followed by 60% feather hydrolysate + 20% peptone + 20% blood, 60% feather hydrolysate + 40% peptone, and 100% blood in that order, while the least growth was observed with 60% feather hydrolysate + 40% blood (Figure 1).
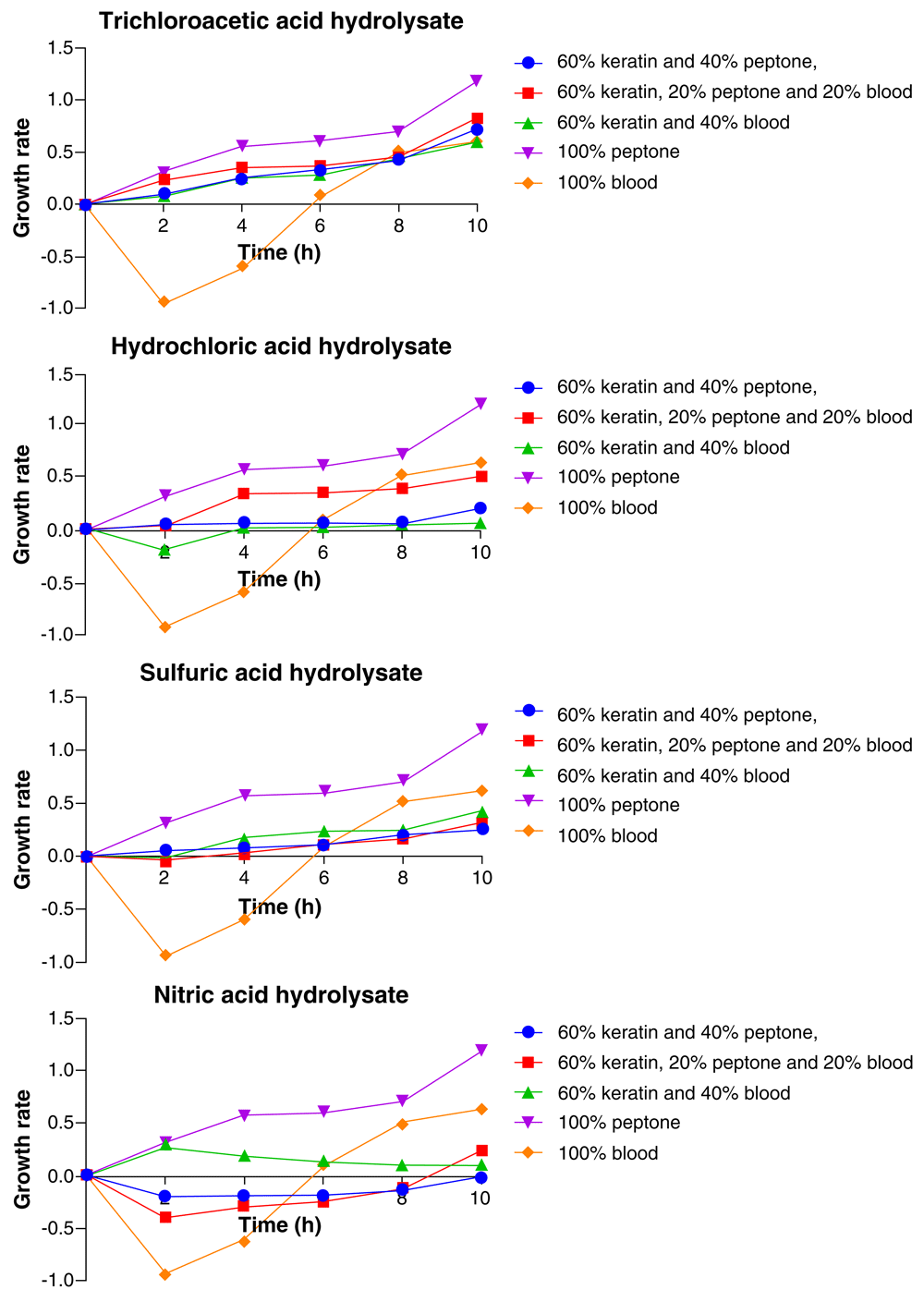
For the HCl feather hydrolysate medium, the growth pattern observed followed the order: 100% peptone > 100% blood > 60% feather hydrolysate + 20% peptone + 20% blood > 60% feather hydrolysate + 40% peptone, while the 60% feather hydrolysate + 40% blood medium showed the least growth. When H2SO4 feather hydrolysate medium was used, the growth was in the order: 100% peptone > 100% blood > 60% feather hydrolysate + 40% blood > 60% feather hydrolysate + 20% peptone + 20% blood, while the 60% feather hydrolysate + 40% peptone was least. In the HNO3 feather hydrolysate medium, C. carpophila grew in the order 100% peptone > 100% blood > 60% feather hydrolysate + 20% peptone + 20% blood > 60% feather hydrolysate + 40% blood, and the lowest growth was recorded in the 60% feather hydrolysate + 40% peptone (Figure 1).
As represented in Figure 2, the highest and the lowest growth of Candida tropicalis varied with medium composition for each of the acid feather hydrolysates used. However, the most frequent medium composition in which the highest growth of the organism was recorded was 100% peptone and the organism showed consistent increase in growth throughout the 10-h period with the highest growth at the 10th hour. When the trichloroacetic acid feather hydrolysate medium was used, C. tropicalis showed no significant difference (p > 0.05) in growth with both 60% feather hydrolysate+ 20% peptone + 20% blood and 60% feather hydrolysate + 40% peptone and the organism’s growth was observed to be highest with both compositions. This was followed by 100% peptone, 60% feather hydrolysate + 40% blood and the least growth was with 100% blood (Figure 2).
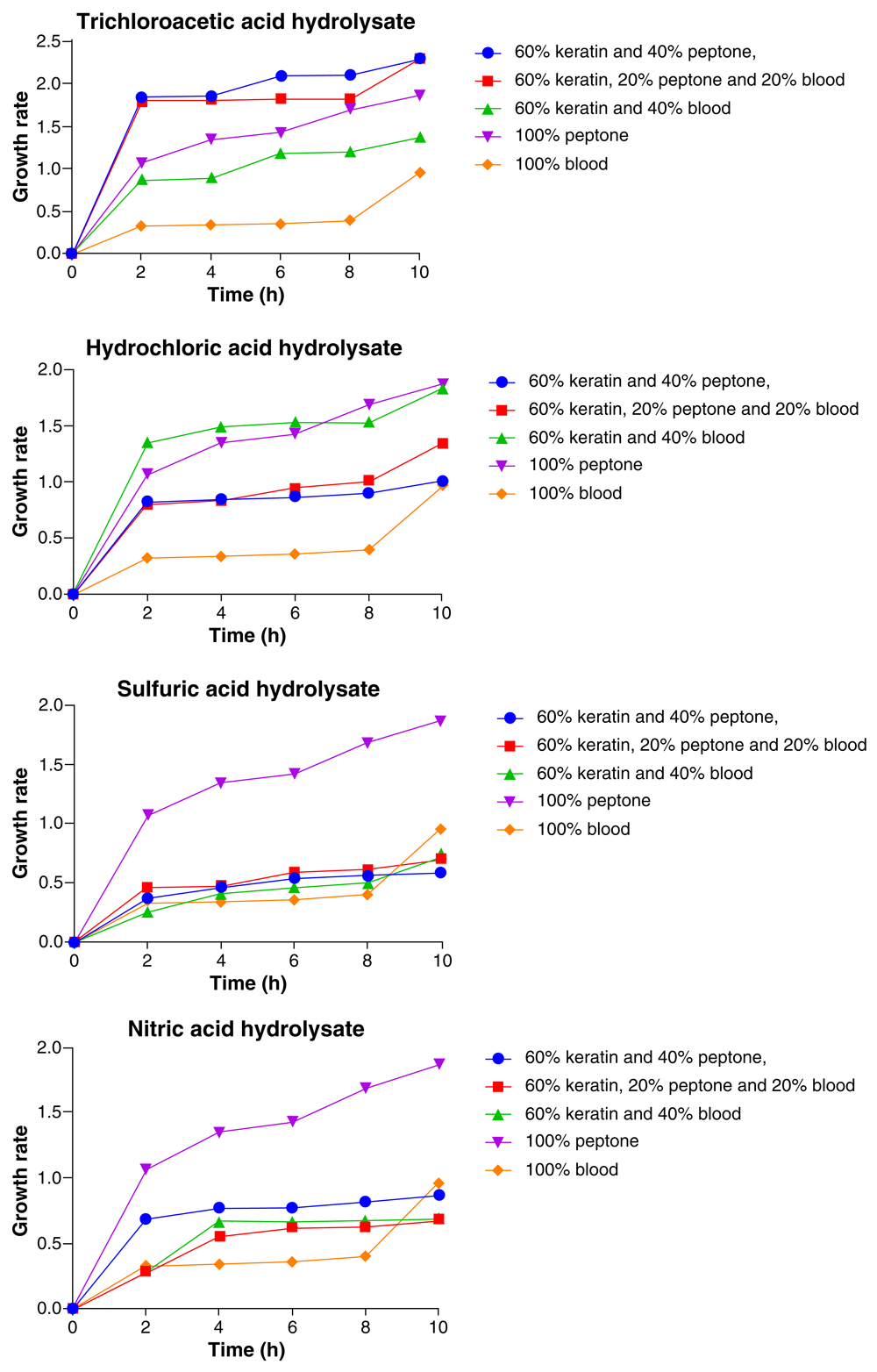
In the HCl feather hydrolysate medium, the growth of C. tropicalis was highest with 100% peptone followed by 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 20% peptone + 20% blood, 60 feather hydrolysate+ 40% peptone, with the lowest growth occurring in the 100% blood medium. For the H2SO4 feather hydrolysate medium, the highest growth of C. tropicalis was observed with 100% peptone, followed by 100% blood, 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 20% peptone + 20% blood and the least growth was observed with 60% feather hydrolysate+ 40% peptone. In the HNO3 hydrolysate medium, C. tropicalis had its highest growth with 100% peptone, followed by 100% blood, 60% feather hydrolysate + 40% peptone, 60% feather hydrolysate + 40% blood, and had its least growth with 60% feather hydrolysate + 20% peptone + 20% blood (Figure 2).
As illustrated in Figure 3, the E. coli showed consistent increase in growth with the highest growth at the 10th hour. The highest and lowest growths of the organism varied with medium composition for all the acid feather hydrolysates. In the TCA feather hydrolysate medium, the highest growth of E. coli at the end of the 10 h period was observed with 60% feather hydrolysate + 40% peptone, followed by 60% feather hydrolysate + 20% peptone + 20% blood, 100% peptone while the least growth was observed with 100% blood and 60% feather hydrolysate + 40% blood. There was no significant difference (p > 0.05) in growth of the organism in both medium compositions. When the HCl feather hydrolysate medium was used, highest growth of the E. coli was with 100% peptone, followed by 60% feather hydrolysate+ 40% peptone, 60% feather hydrolysate + 20% peptone + 20% blood and 60% feather hydrolysate + 40% blood while the least growth was with 100% blood (Figure 3).
For the H2SO4 feather hydrolysate medium, E. coli had its highest growth with 100% peptone followed by 60% feather hydrolysate + 40% peptone, and then 60% feather hydrolysate+ 40% blood and 60% feather hydrolysate + 20% peptone + 20% blood in which there was little or no significant difference in growth while the least growth of E. coli was recorded with 100% peptone. In the HNO3 feather hydrolysate medium, the highest growth of E. coli recorded was with 60% feather hydrolysate+ 40% peptone, followed by 100% peptone, while the least growths were observed with 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 20% peptone + 20% blood and 100% blood, all showing no significant difference (p > 0.05) in growth (Figure 3).
The results in Figure 4 showed consistent increase in growth rate of Klebsiella pneumoniae throughout the 10 h period with highest growth at the 10th hour. The highest and lowest growths of the organism varied with the medium composition. In the trichloroacetic acid feather hydrolysate medium, the highest growth of Klebsiella pneumoniae was with 100% blood, followed by 60% feather hydrolysate + 40% peptone, 60% feather hydrolysate + 40% blood, 100% peptone, and the least growth was with 60 feather hydrolysate + 20% peptone + 20% blood. In the HCl feather hydrolysate medium, the Klebsiella pneumoniae had its highest growth with 100% blood, followed by 60% feather hydrolysate+ 40% peptone, 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 20% peptone + 20% blood, and the least growth was with 100% peptone (Figure 4).
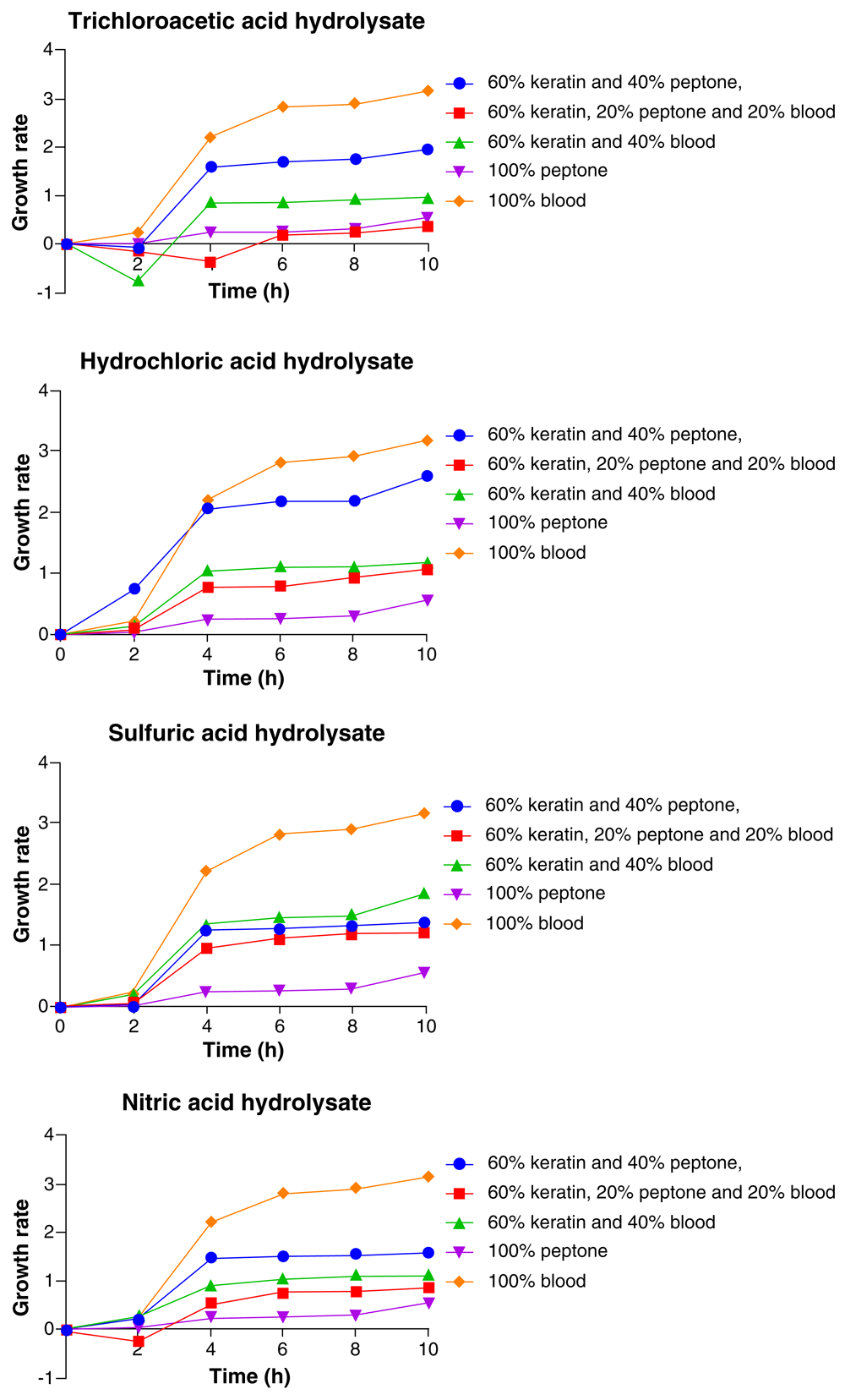
In the H2SO4 feather hydrolysate medium, the medium composition in which Klebsiella pneumoniae had its highest growth was 100% blood, followed by 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 40% peptone and then, 60% feather hydrolysate + 20% peptone + 20% blood, while the least was recorded in 100% peptone medium. In the HNO3 feather hydrolysate medium, the highest growth of Klebsiella was observed in 100% blood, followed by 60% feather hydrolysate + 40% peptone, 60% feather hydrolysate + 40% blood, 60% feather hydrolysate+ 20% peptone + 20% blood and the least growth of the organism was observed in 100% peptone (Figure 4).
As represented in Figure 5, Pseudomonas aeruginosa showed consistent increase in growth for the 10 h period and the highest growth was recorded at the 10th hour. The medium composition which yielded the highest growth of the organism was 100% peptone and the least growth was recorded with 100% blood medium. This was constant for all the acid hydrolysate medium. In the TCA feather hydrolysate medium, the medium composition after 100% peptone that yielded the highest growth of P. aeruginosa was 60% feather hydrolysate+ 40% peptone, followed by 60% feather hydrolysate + 20% peptone + 20% blood, 60% feather hydrolysate + 40% blood, while the least growth of Pseudomonas aeruginosa was with 100% blood (Figure 5).
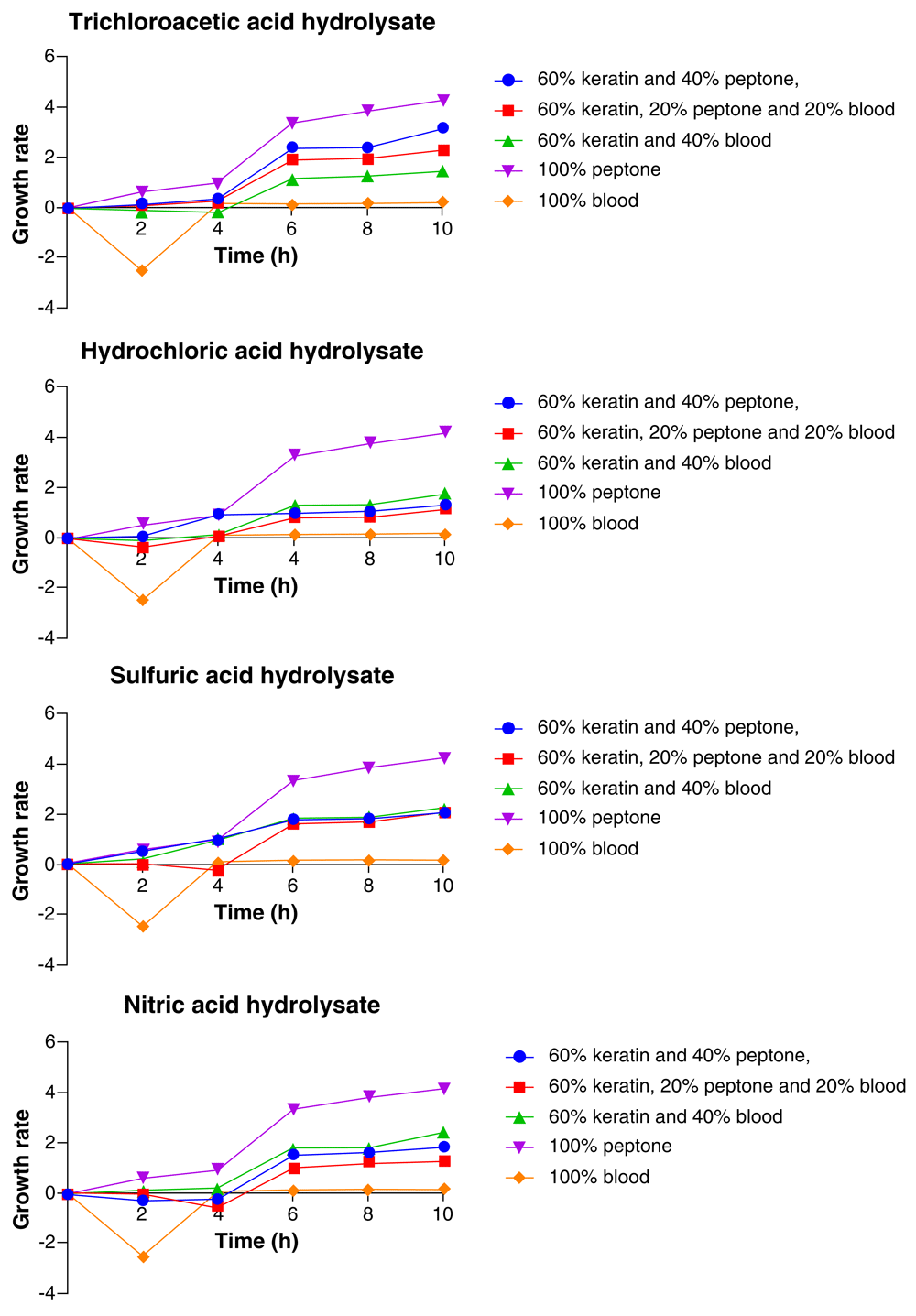
In the HCl feather hydrolysate medium, after 100% peptone, P. aeruginosa had its highest growth with 60% feather hydrolysate + 40% blood, followed by 60% feather hydrolysate + 40% peptone, 60% feather hydrolysate + 20% peptone + 20% blood, while the organism had its least growth with 100% blood. In the H2SO4 feather hydrolysate medium, 100% peptone remained the medium composition in which the highest growth of P. aeruginosa was recorded, followed by 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 40% peptone and 60% feather hydrolysate + 20% peptone + 20% blood while the least growth was observed in 100% blood. In the HNO3 feather hydrolysate medium, following 100% peptone, the highest growth of P. aeruginosa was observed with 60% feather hydrolysate+ 40% blood, followed by 60% feather hydrolysate + 40% peptone, 60% feather hydrolysate + 20% peptone + 20% blood. The least growth was observed with 100% blood medium (Figure 5).
Figure 6 shows the growth pattern of Pichia kudriavzevii in the different medium. Pichia kudriavzevii showed consistent increase in growth with the highest growth at the 10th hour. The highest growth of the organism was 100% peptone medium, while the least growth was observed in 100% blood medium. In the TCA feather hydrolysate medium, the order of growth of Pichia kudriavzevii from the highest to the least in the medium compositions was 100% peptone > 60% feather hydrolysate + 40% peptone > 60% feather hydrolysate + 40% blood > 60% feather hydrolysate, + 20% peptone + 20% blood >100% blood (Figure 6).
In the HCl feather hydrolysate medium, the order of growth was: 100% peptone > 60% keratin + 40% peptone > 60% feather hydrolysate + 40% blood > 60% feather hydrolysate + 20% peptone + 20% blood > 100% blood. In the H2SO4 feather hydrolysate medium, the growth of the organism followed the order: 100% peptone > 60% feather hydrolysate + 40% blood > 60% feather hydrolysate + 40% peptone > 60% feather hydrolysate + 20% peptone + 20% > 100% blood. In the HNO3 feather hydrolysate medium, the order of growth in the medium compositions was: 100% peptone > 60% feather hydrolysate + 40% blood > 60% feather hydrolysate + 40% peptone > 60% feather hydrolysate + 20% peptone + 20% blood > 100% blood (Figure 6).
The growth rate of the Proteus mirabilis in the different medium is shown in Figure 7. As shown in the Figure, the highest growth was recorded at the 10th hour for all the medium compositions against the respective acid hydrolysates
In the TCA feather hydrolysate medium, besides 100% peptone, in which the highest growth was recorded, the second highest growth of Proteus mirabilis was with 100% blood medium, followed by 60% feather hydrolysate + 40% peptone, 60% feather hydrolysate + 40% blood and the least growth was with 60% feather hydrolysate + 20% peptone + 20% blood. In the HCl feather hydrolysate medium, after 100% peptone, the highest growth of Proteus mirabilis was observed with 100% blood, followed by 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 20% peptone + 20% blood, and the least growth was with 60% feather hydrolysate + 40% peptone (Figure 7).
In the H2SO4 feather hydrolysate medium, after 100% peptone, Proteus mirabilis growth pattern followed the order 100% peptone > 60% feather hydrolysate + 40% peptone > 60% feather hydrolysate + 40% blood 60% feather hydrolysate + 20% peptone + 20% blood (Figure 7).
In presence of the different hydrolysates, Staphylococcus aureus showed consistent increase in growth throughout the period of incubation. The 100% peptone medium produced the best growth against all the acid hydrolysate medium, while the least growth of the organism varied with medium composition.
In the TCA feather hydrolysate medium, the 60% feather hydrolysate + 40% peptone medium was second to the 100% peptone medium in supporting the growth of Staphylococcus aureus, followed by 60% feather hydrolysate + 20% peptone + 20% blood, 100% blood and 60% feather hydrolysate + 40% blood medium in that order. In the HCl feather hydrolysate medium, the second highest growth of the Staphylococcus aureus was observed with 60% feather hydrolysate + 40% blood, followed by 100% blood, 60% feather hydrolysate + 40% peptone, and 60% feather hydrolysate + 20% peptone + 20% blood in that order (Figure 8).
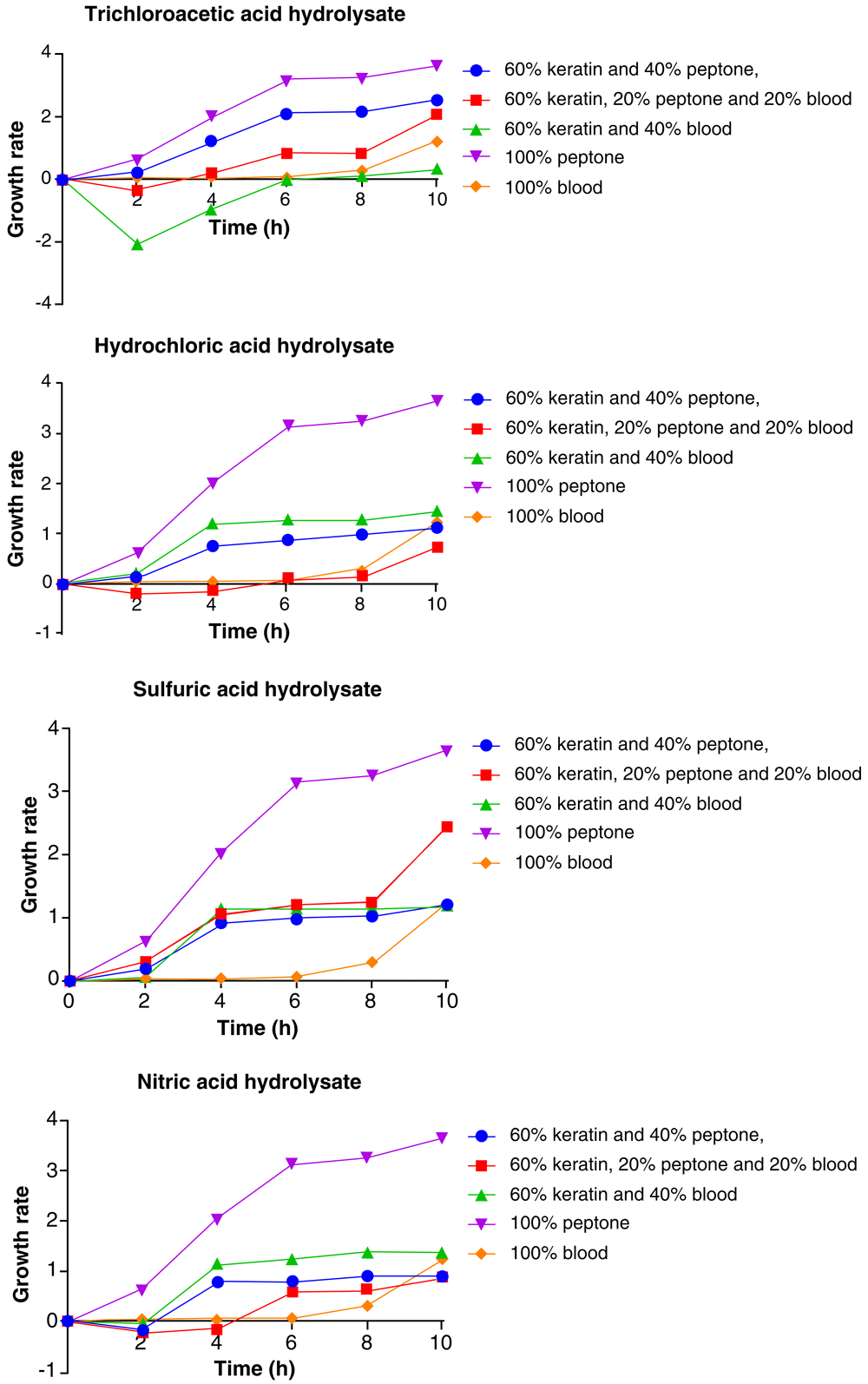
In the H2SO4 feather hydrolysate medium, the 60% feather hydrolysate+ 20% peptone + 20% blood medium ranked second to the 100% peptone medium in the observed growth pattern for Staphylococcus aureus, followed by 60% feather hydrolysate + 40% blood, 60% feather hydrolysate + 40% peptone, and 100% blood medium in that order. In the nitric acid feather hydrolysate medium, the growth pattern observed for Staphylococcus aureus followed the order 100% peptone > 60% feather hydrolysate + 40% blood > 60% feather hydrolysate + 40% peptone > 60% feather hydrolysate + 20% peptone + 20% blood (Figure 8).
In this study, the chicken feather keratin peptone was obtained by alkaline hydrolysis and acid neutralization and precipitation delete. In most cases KOH, NaOH and Ca(OH)2 are used for the hydrolysis. Taskin et al., (2016) used the alkaline hydrolysis method with KOH in a study where peptone was obtained from sheep wool protein hydrolysate. From investigations, alkaline hydrolysis was reported to be able to produce a high yield of keratin and also enhance the keratin extraction effectiveness (Sinkiewicz et al., 2017). Alkaline hydrolysis has also been studied and proven to be effective in the degradation of waste containing keratin and collagen (Gousterova et al., 2005). It is opined that the use of alkaline hydrolysis inactivates of pathogens and prions such as transmissible spongiform encephalopathy (TSE) and bovine spongiform encephalopathy (BSE). The use of alkaline hydrolysis yields a BSE and TSE free hydrolysate medium (Matthews & Cooke, 2003). Despite the fact that heat is employed in most chemical hydrolysis processes to improve yield, the alkaline hydrolysis in this study was carried out under room temperature for approximately 10 h. Chemical hydrolysis conducted at high temperatures is said to lead to the destruction of amino acids (Sinkiewicz et al., 2017).
Although acid hydrolysis has also been used in some studies to obtain protein hydrolysates (Wisuthiphaet & Kongruang, 2015), it is argued that it could result in destruction of essential amino acids, such as methionine, cystine, cysteine and tryptophan, and conversion of glutamine and aspargine to glutamic and aspartic acid, respectively. It is indicated that during acid hydrolysis, the salts in hydrolysates can be injurious to the growth of the microorganisms (Bucci & Unlu, 2000).
This study aimed to assess a cheaper source of protein for microbial culture than conventional nutrient medium. Chicken feathers were chosen as the material for research because of its abundance, cost-efficiency and high protein content. The results obtained in this study with reference to the performance of the organisms in the formulated medium compositions were viewed in comparison with the performance of the organisms in the commercially produced peptone. The comparison of the growth rate of the organisms in the different medium with that in peptone was deliberate. Studies have revealed peptone as an excellent nitrogen and protein source for growing microorganisms and manufacture of growth medium (Markings, 2018).
The results of the present experiment showed that in most cases, the organisms growth was highest with the 100% commercially produced peptone while in other cases, various medium compositions yielded higher growth of the organisms even better than in the 100% peptone.
The medium compositions that favored the highest growth of the organism differed with each acid hydrolysate for every organism. The best growth of Candida carpophila was recorded with the commercial peptone irrespective of the acid hydrolysate medium composition used. However, in the case of Candida tropicalis, the formulated medium compositions from the chicken feather hydrolysate were able to yield higher growths of Candida tropicalis even better than the commercial peptone but only in the TCA hydrolysate, while the commercial peptone yielded the highest growth of the organisms in the other acid hydrolysates. Pseudomonas aeruginosa, Pichia Kudriavzevii, Proteus mirabilis and Staphylococcus aureus consistently had their highest growth with the commercial peptone irrespective of the acid hydrolysate used. In Escherichia coli and Klebsiella pneumoniae, variation of the highest growth with the hydrolysate medium compositions and growth yield of the organisms higher than in the commercial peptones persisted. The variations in the medium compositions that showed the best growth of the organisms were indications that the acids used in precipitating the keratin from the chicken feathers had significant impact on the efficacy of the medium compositions. The optimum concentration of chicken feather keratin concentration that yielded maximum microbial growth was 60% feather hydrolysate + 40% peptone in Escherichia coli.
The ideal chicken feather hydrolysate that yielded microbial growth was the TCA hydrolysate. In a study by Taskin et al. (2016), using sheep wool protein hydrolysate as a peptone source for microorganisms, Staphylococcus aureus was observed to show poor growth performance in the medium. However, growth performances of Saccharomyces cerevisiae, Bacillus subtilis and Penicillium chrysogenum were observed to be moderate in wool protein. Generally, the present study revealed that growth rate of the respective organisms varied from one medium composition to the other. This observation has been reported by earlier workers (Aspmo et al., 2005; Jones & Fayerman, 1987).
Waste chicken feathers that were previously discarded and viewed as a burden to the environment can now be viewed as an important bio-resource and can be manipulated and explored widely as a biotechnological material. This study was able to characterize waste chicken feathers as a microbiological tool for microbial culture. Because of its high protein content, protein essential for the growth of microorganisms can be synthesized from chicken feathers.
In the study, the potential of the chicken feather keratin to support the growth of both bacteria and fungi was established. Chicken feather hydrolysate was also proven to be an excellent substrate particularly for the growth of Escherichia coli. Using a suitable process of hydrolysis, peptone from chicken feather keratin can be re-modified, industrialized and produced in bulk on a commercial scale. This research work could serve as precursor to exploring many other waste materials of high protein content which could be of biotechnological value.
The raw absorbance values from analysis of microbe growth using different growth media are available on figshare. DOI: https://doi.org/10.6084/m9.figshare.7376564.v1 (Akpor et al., 2018).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Brandelli A, Sala L, Kalil S: Microbial enzymes for bioconversion of poultry waste into added-value products. Food Research International. 2015; 73: 3-12 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Microbial biotechnology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: biomaterials, biopolymers, proteins, waste management
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 07 Aug 19 |
read | ||
|
Version 2 (revision) 30 Jan 19 |
read | read | read |
|
Version 1 10 Dec 18 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)