Keywords
primary forest, secondary forest, shrub swamp, swamp forest, community ecology, forest disturbance, forest fires, selective logging
This article is included in the Ecology and Global Change gateway.
primary forest, secondary forest, shrub swamp, swamp forest, community ecology, forest disturbance, forest fires, selective logging
We are losing tropical forests and their associated biodiversity worldwide to deforestation, and this loss is irreplaceable1. Forest loss also occurs because of degradation: selective logging affects large areas2, which in turn become more susceptible to other disturbances like fire3. Most studies focus on the Amazon region, but forests in Southeast Asia also face great threats4. Forest losses due to fire have recently gained more attention in Indonesia5,6. Indonesia has the highest deforestation rate of all countries7, and even protected forests suffer losses8. The effectiveness of protecting forests in Indonesia has been questioned for Kalimantan9, but for Sumatra, modest progress has been made, especially as large-scale logging has slowed down10.
The impacts of forest loss and degradation on biodiversity are better known in the Amazon region, for instance for birds11. In Southeast Asia, most primary forest bird species still occur in previously logged forests12–14. Globally, bird feeding guilds respond differently to disturbance15, but forest understory insectivores were identified as the most sensitive16. The severity of the disturbance and its impact on soils largely determine the duration of recovery, while the surrounding landscape acts as a source of biota17.
We lack studies investigating the impact of disturbances - from logging or fire - on bird communities in peat swamp forests, despite their crucial importance for biodiversity, flood control, carbon stocks, potential greenhouse gas emissions, and their high vulnerability to drainage and fires18,19. Few studies have focused on the potential benefit of disturbances for overall landscape biodiversity apart from theoretical and modelling approaches20,21.
In this paper we describe the bird communities in a tropical peat swamp on Sumatra, Indonesia. Bird surveys of the Berbak area date far back22,23. We sampled birds in three habitats defined according to Keddy (2010)24: primary swamp forest, secondary swamp forest resulting from selective logging, and shrub swamp resulting from forest fire. We compare species richness and bird communities between these habitats both taxonomically (species richness, abundance) and functionally (vocalisation activity, body mass, wing length, distribution area). We ask the question whether disturbances increase the overall diversity of the landscape. We discuss the implications of our results for maintaining the overall bird diversity of the peat swamp and for bird conservation in Berbak.
We surveyed birds in Berbak National Park, a peat swamp situated in the province of Jambi, on the east coast of the island of Sumatra in Indonesia (Figure 1). Berbak National Park is a Ramsar site25 and an Important Bird Area26. A severe drought in 1997 facilitated forest fires which were aided by human disturbance due to natural rubber collection (Dyera costulata). The burned areas subsequently developed into shrub swamp. Tree stumps also reveal that illegal selective logging affected several areas of the park, resulting in secondary forest habitats.
We chose 12 sites for counting birds, divided in four sites for each of three habitat types of the peat swamp area, representing different past disturbance levels: primary forest (no disturbance), secondary forest (selective logging), and shrub swamp (fire).
Field surveys.
We recorded audible sound in all 12 sites. We used autonomous sound recorders (SM2+ recorders with SMX-II microphones, Wildlife Acoustics Inc.) and did not carry out visual surveys, since acoustic recording constitutes a valid survey method for assessing bird richness27, especially for cryptic birds in tropical forests. From February to November 2013, we sampled all three habitats at one site each month for 48 hours, starting at midnight. Before the recording started, we counted and measured the diameter at breast height (DBH) of all trees with a circumference above 20 cm (diameter at breast height of ~6.4 cm) inside an area of 14 × 14 m delimited by spanning 10 m coloured ropes from the central tree where the sound recorder was attached to all cardinal directions. We also recorded whether the site was flooded or not at the time of the sound recorder installation.
We uploaded 20 minutes recordings after sunrise for each plot in each month (24 recordings in total) to our online platform (http://soundefforts.uni-goettingen.de/). The provenance of the recordings was hidden and all birds within the sound recordings were identified by author IF. The distance of bird vocalisations was estimated by ear to the meter, based on their loudness in the sound recording compared to the ambient sound level and knowledge of the source sound level of each species. The start and end time of each vocalization was recorded to compute the duration of the bird vocalisations. We complemented our data set with species-specific information about the feeding guild28,29, body mass data28,30, wing length data31, the IUCN Red List threat status32 and distribution area33 to analyse conservation and functional aspects of bird community differences.
The data were analysed in R 3.4.3 and graphs were generated with the package ggplot234. We excluded detections that were not identified to species, as well as detections above 50 m to compare sites at a common detection radius35. We computed species richness, bird vocalizing activity in minutes, and bird abundance per acoustic count (i.e. recording). Bird species richness was further computed at the site and habitat level. Bird abundance was counted as the sum of the maximum number of individuals vocalising simultaneously in each species. We calculated community-weighted means (or community functional parameter36, hereafter CWM) for body mass, wing length, and distribution area for each count. Due to microphone failure, the second recording of one of the forest plots had only one audible audio channel and was therefore removed from the count-level analysis.
At the count level, the species richness and abundance between habitats was modelled using generalised linear mixed effects models of the poisson family (lme4 package37), with site as random variable, and we checked that the models were not over-dispersed. Similarly, vocalizing activity was analysed with a linear mixed effects model. At the site level, we used generalised linear models of the poisson family to model alpha and beta bird species richness, which we calculated using the additive partitioning approach38. For all models, we used tree number, tree basal area, and habitat type as predictors. We generated all possible predictor combinations and compared the models using Akaike's Information Criterion for small sample sizes (herafter AICc, MuMIn package, dredge function) to choose the best model (with the lowest AICc).
We visualized the composition of the bird communities in non-metric multidimensional scaling graphs generated with the package vegan39, and tested the significance of the habitat in structuring these communities with an ADONIS test40. We also plotted the abundance of birds within different families in each of the habitats along with their conservation status. To investigate whether the combined, different habitats lead to higher species richness than one primary forest area of similar size, we calculated the rarefied richness based on the entire bird community, rarefied to 4 sampling sites, to compare it to the number of species found in the 4 forest sites.
We detected 379 birds overall, belonging to 90 species (Table S1). Among those, 26 individuals were not identified to species and 2 were detected above 50 m, resulting in a working dataset of 351 detections. The three habitats differed considerably based on their vegetation structure and the distribution of their DBH values (Figure S1 and Figure S2).
Species richness and abundance at the count level, and mean alpha and beta species richness at the site level were similar between the habitats (Figure 2 and Figure 3).
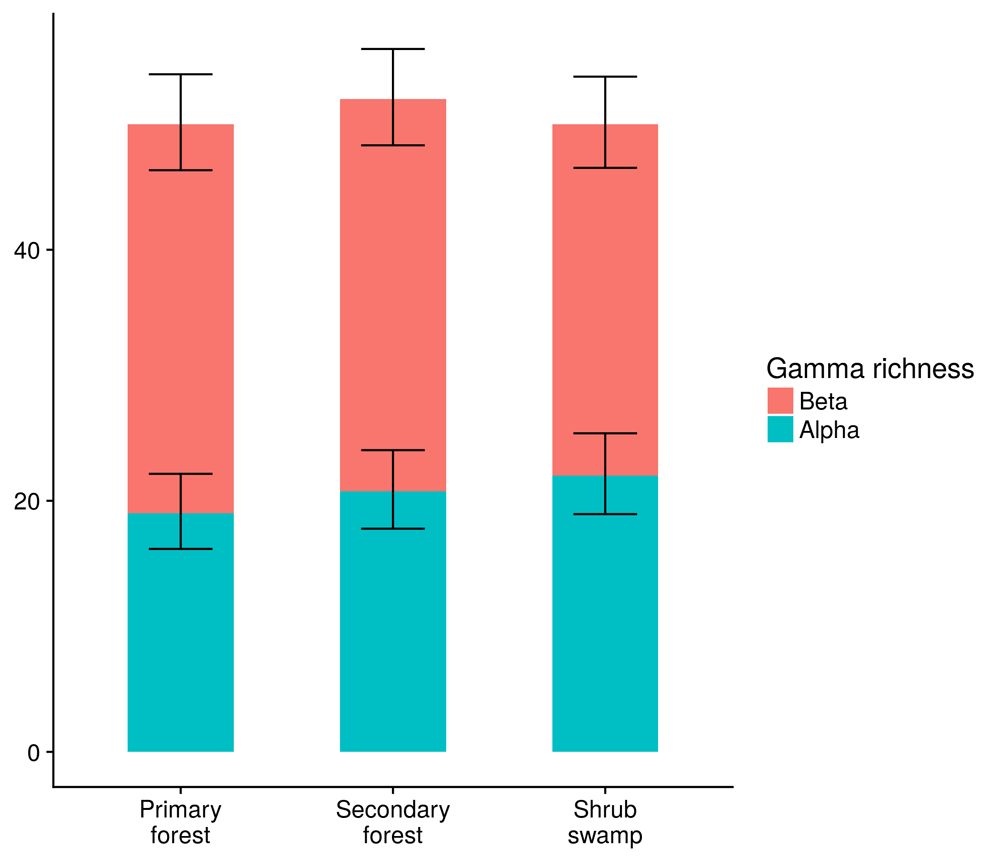
Total (gamma) richness for each habitat type, split by alpha and beta richness components. Error bars show the 83% confidence intervals for the mean alpha and beta richness values. Overlapping bars indicate that the means are not significantly different.
These variables were best explained by null models. Gamma species richness per habitat was as follows: primary forest: 50 species, secondary forest: 52 species, bush swamp: 50 species. Bird richness and abundance between habitats, split into different functional groups and IUCN red list threat categories, were similar between habitats (Figure S2).
Bird vocalisation activity at the count level was similar among habitats (Figure 3) and best explained by a null model. CWMs of body mass, wing length and distribution area however were increasing along the disturbance gradient and the CWMs in shrub swamp were significantly higher than in primary forest. Wing length CWM was also higher in shrub swamp than in secondary forest (Figure 3).
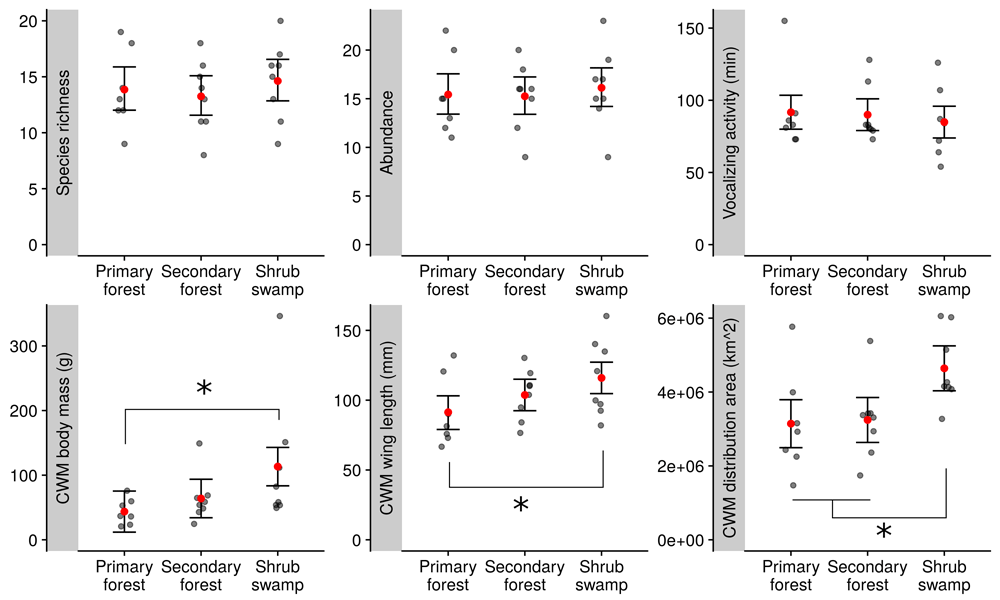
Bird species richness, abundance, vocalizing activity, community-weighted mean (CWM) of body mass, wing length, and distribution area33 per count in three different habitats of the peat swamp of the Berbak national park. Mean values are represented with red dots and their 83% confidence intervals are indicated with error bars. Means are significantly different when their confidence intervals do not overlap, and significant differences are indicated with asterisks.
Bird communities differed greatly between primary forest and bush swamp, with secondary forest being an intermediate habitat having overlap with both of the latter (Figure S3). The ADONIS test revealed that habitat structured bird community composition with marginal significance (P=0.053). The difference in the bird communities arose mainly from the higher abundance of Timaliidae (Old World babblers) in the forest habitats. Pycnonotidae (Bulbuls) and Alcedinidae (Kingfishers) were more prevalent in shrub swamp, while Nectarinidae were more frequent in the forest habitats (Figure 4).
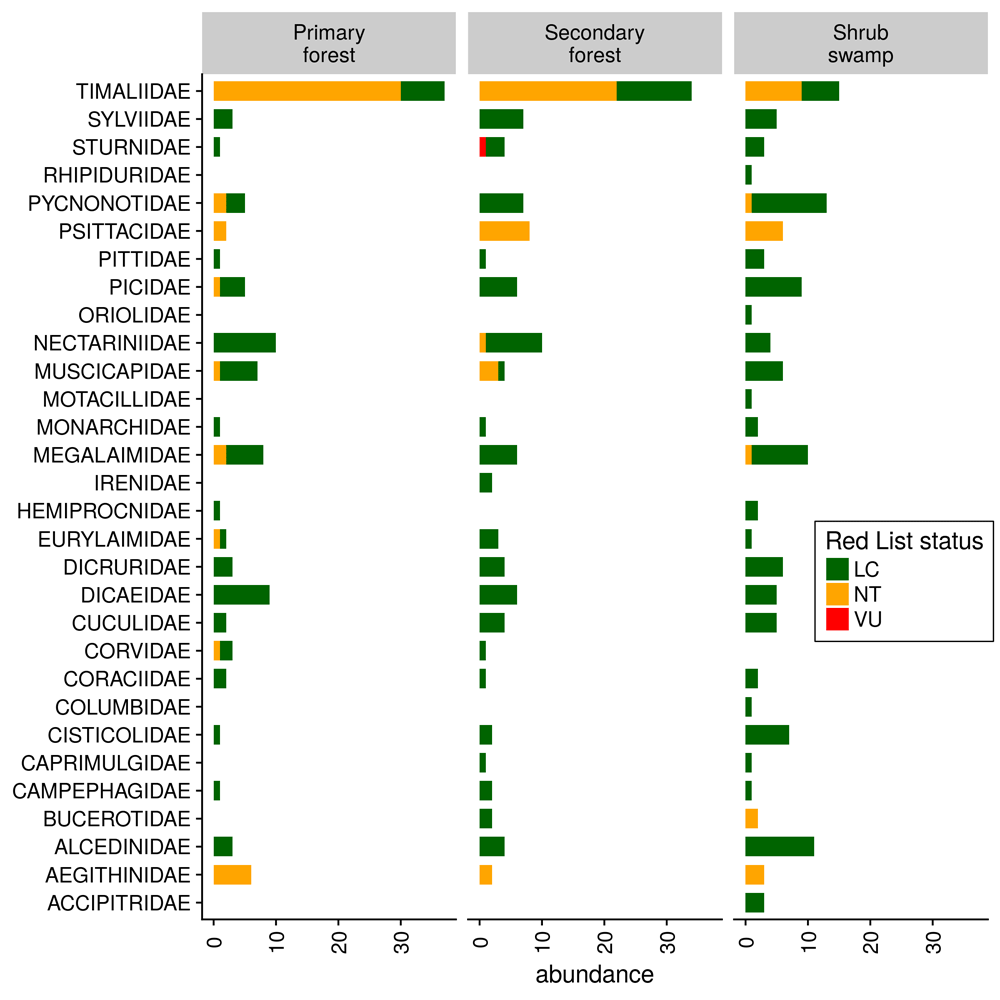
Abundances are split by habitat and shown for different IUCN Red List statuses.
We have shown that all three habitats inside the Berbak peat swamp forest have similar abundance, vocalizing activity and species richness. The habitat, tree number or basal area were not related to these measures. Even the richness and abundance of the bird feeding guilds and abundances inside families were similar between habitats. However, we detected differences in the bird community composition, notably a decrease in understory insectivores (Old World Babblers, Timaliidae) which was compensated by the appearance of other species in shrub swamp.
The Berbak peat swamp forest is threatened from many sides. It is difficult to access as only waterways (irrigation canals and rivers) lead into it, and large parts are temporarily flooded due to tides. However, wild bird extraction for the caged bird market, illegal logging, land-use conversion at its margins, and natural rubber (jelutung) collection are all currently happening41. These human activities increase the risk of fires, especially during dry spells caused by the warm phases of the El Niño Southern Oscillation, which led to the especially severe fires of 1994, 1997, and 2015 (after our survey).
Primary forest sites had high conservation value because we found 16 species of conservation concern ("near threatened" status according to IUCN Red List status), twice as much as in shrub swamp. More acutely threatened, rare species might be detected with higher sampling effort by processing more recordings. Secondary forest sites were exactly intermediary with 12 species of conservation concern, although one bird, the Javan Myna (Acridotheres javanicus), has recently been classified as vulnerable. Javan Mynas are commonly sold on the market in Jambi city. The absence of hornbills in primary forest seems fortuitous, as we also detected one Buceros rhinoceros in forest also, but at an estimated distance of 60 m. Compared to the secondary forest sites surveyed by Prabowo et al.42 in the same province (Harapan rainforest) and year, the detected bird abundance was much higher in Berbak (4 birds per count in Prabowo et al. versus 13 birds per count in the present study for the same detection radius). Our secondary forest sites seemed relatively well-preserved, as we could not detect any typical loss of understory and terrestrial insectivores due to changes in understory vegetation43. Considering that the detected abundance of birds in the other habitats was similarly high, this indicates that the National Park of Berbak provides relatively good living conditions for birds, and especially conservation-worthy species thrive in the primary forest tracts.
Nevertheless, bird communities differ between habitats as was shown in the non-metric multidimensional analysis (Figure S4). It turned out that the differences in bird communities arose mainly from the absence of Timaliidae in shrub swamp. Timaliidae are mainly understory insectivores, which are generally recognised to be sensitive to forest disturbance and thus act as indicator species44. The higher prevalence of generalist bulbuls and open-area kingfishers in shrub swamp is also typical of disturbed habitats in the region, such as oil palm plantations42. Notably, sunbirds were almost absent in shrub swamp, which does not seem to provide enough floral resources (pers. obs. KD). Interestingly, the species in shrub swamp were heavier, had longer wings and wider distribution ranges, indicating they may be more mobile, widespread species that are less of a conservation concern; these features are also typical of generalist species. The higher body masses may arise from the prevalence of kingfishers and bulbuls, which are relatively big.
The different habitats lead to a high diversity at the national park level: while the total species count per habitat was almost identical (around 50), the overall species richness reached 89 species. It is tempting to conclude that the heterogeneity introduced by disturbances such as logging or fires (which created these different habitats) increased bird species richness. However, in comparison to an equally large area consisting only of primary forest, the combination of different habitats does not lead to a markedly higher species richness, as we found a nearly identical rarefied species richness of 51 for all habitats combined. The fact also remains that primary forest harboured a higher proportion of conservation-worthy bird species, while generalists were more prevalent in the shrub swamp.
Our autonomous acoustic sampling protocol gathered many more data than we analysed so far: we only processed around 0.7% of the available data, albeit the most promising dawn choruses. We would determine a higher proportion of the bird community with random or carefully chosen time windows throughout the day45. We welcome interested prospective co-authors to process more recordings to complete the species list of the still only superficially studied avifauna of Berbak. Repeated surveys to the same sites, after the major fires from 2015, would also yield insights into how bird populations are changing in the longer term in response to these and other disturbances from wildlife trade.
Dataset 1: The data and R script required to reproduce our graphs and results are provided. This file contains: Berbak birds.csv contains the bird detection data; Berbak vegetation.csv contains the individual DBH values for all plots; Berbak birds analysis.R is the R script that performs the analysis and graphing. DOI, 10.5256/f1000research.13996.d19550546.
The source audio material is available at http://soundefforts.uni-goettingen.de/biosounds/collection/show/3.
This research was funded by the German Science Foundation (DFG) with the grant number SFB990/1.
This study was financed by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the collaborative German - Indonesian research project EFForTS (CRC990). We thank the Foreign Research Permit Ministry of Research Technology and Higher Education of Indonesia for granting the permit to KD (211/SIP/FRP/SM/VI/2012). The National Park of Berbak authorities granted us access to the Berbak swamp forest and the Zoological Society of London allowed this scientific collaboration. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University.
Table S1: Detected bird numbers in each habitat.
Click here to access the data.
Figure S1: Examples of sampling sites.
1: BB3 shrub swamp with burned tree stump; 2: BP2 primary swamp forest; 3: BS2: secondary swamp forest with installed sound recorder and coloured ropes for the vegetation survey.
Click here to access the data.
Figure S2: Distribution of tree sizes in the different habitats.
We measured the diameter at breast height (DBH) of all trees with a DBH above approximately 6.4 cm (circumference of 20 cm).
Click here to access the data.
Figure S3: Species richness per habitat and IUCN Red List threat status.
Click here to access the data.
Figure S4: Non-metric multidimensional scaling of bird communities in the different habitats.
Brown represents shrub swamp, light green secondary forest, dark green primary forest.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Conservation biology, bioacoustics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Conservation biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 14 May 18 |
read | read |
|
Version 1 26 Feb 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)