Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic and adherent cells characterized by high CD90, CD105 and CD73, but lack of CD14, CD34 and CD45 expression. When treated with certain differentiation-stimulating factors, these cells will differentiate into adipocytes, chondrocytes, as well as osteocytes. MSCs show clinical feasibility, as studies on multiple animal models that yielded therapeutic efficacy gave rise to a series of clinical trials in a wide range of major diseases1. Umbilical cord and adipose tissue are two of the most common sources of MSCs, due to the fact that they can be non-invasively obtained and have minimal risk in terms of immunological and ethical issues. Apart from sharing the common properties of MSCs, umbilical cord-derived stem cells (UCSCs) are reported to have higher proliferation capability than adipose-derived stem cells (ASCs), whereas ASCs are able to differentiate to adipose tissue better than UCSCs2. Very recently, we have demonstrated that both ASCs and UCSCs expressed lower ALDH1A1 and OCT-4 than breast cancer stem cells (BCSCs), indicating that both types of MSCs have lower pluripotency compared to BCSCs (Wanandi SI, Purnamawati, Tamara A, Putri KT, Simadibrata D, in press).
Nowadays, MSCs are widely used for regenerative therapies due to their multipotent differentiation capacity. On top of that, MSCs are capable to secrete various paracrine signals for tissue regeneration and revascularization, such as chemoattractive, immunomodulatory, angiogenic, anti-apoptotic, and pro-survival factors. Nevertheless, MSC secretomes have also been reported to promote cancer progression and metastasis3. Accumulating evidence of cell-cell and paracrine interactions between MSC and cancer cells has indicated that MSCs can either induce or inhibit tumor progression3–9. Secretomes contained in conditioned medium (CM) of MSCs consist of various biologically active factors that have the same effectiveness as MSCs themselves10. In addition, secretomes of MSCs are considered safer to use since they do not contain cellular elements,11 making them free from possible mutations and transformation into cancer-associated fibroblast in a cancer microenvironment12.
Normal and cancer stem cells share similar properties, such as self-renewal capacity and differentiation potential into multiple cell types. Activity of aldehyde dehydrogenase (ALDH) superfamily have been widely used as a marker of viable normal stem cells, as well as cancer stem cells13. In breast cancer, the existence of ALDH+ BCSCs often correlates with poor prognosis, progression, chemoradiation resistance, and metastasis. Chemotherapy resistance is due to the role of ALDH as a detoxifying enzyme, which mediates detoxification of toxic aldehyde intermediates that are produced in certain chemotherapeutic agents-treated cancer cells, while radioresistance occurs through direct removal of oxygen radicals and indirect production of antioxidant compound nicotinamide adenine dinucleotide (phosphate)14.
ALDH1A1 and ALDH1A3, two of ALDH1 superfamily isozymes, have been known to play important roles in the modulation of the retinoic acid signaling pathway, which can either support or suppress cancer growth14–16. In addition, they also serve as markers of stemness in CSCs13,17. The expression of ALDH1A1 was associated with advanced, triple-negative, and poor prognosis breast cancer after neoadjuvant chemotherapy18,19. ALDH1A1 expression was also found to be predictive of tumor responsiveness to cyclophosphamide treatment20,21. Meanwhile, the isoform of ALDH1A3 has been suggested to promote breast cancer progression via retinoic acid signaling22.
TGF-β family members play a major regulatory role in various biological and physiological functions23. TGF-β physiologically exerts anticancer activities in normal and benign cells by prohibiting cell proliferation and by creating cell microenvironments that inhibit cell motility, invasion, and metastasis. However, along with the development and progression of tumors, various mutations or deletions occur in genes that encode various TGF-β signaling components. These lead to the loss of protective and cytostatic effects of TGF-β, which in turn alters TGF-β signaling to promote cancer progression, invasion, and tumor metastasis23,24. Recently, the mechanism of the TGF-β paradox may have been explained through Erk activation, which will auto-induce TGF-β in the microenvironment25. Auto-induction of TGF-β in benign or early stage cancer cells will create negative feedback of TGF-β signaling leading to growth arrest, whereas progression and metastasis in malignant cancer cells will be induced via a positive feedback loop25.
Until now, the interaction between MSC secretomes and BCSCs has not been fully understood. Our recent study has indicated that BCSCs treated with secretomes of ASCs expressed ALDH1A1 significantly higher compared to those treated with UCSCs, in accordance to OCT-4 and SOX2 expressions26. Those results showed that secretomes of ASCs contribute to pluripotency and viability of BCSCs more than those of UCSCs. To gain a better understanding of the effects of MSC secretomes on BCSCs, we conducted the present study on ALDH1A3 expression in association with TGF-β1 signaling pathways in BCSCs.
Methods
Ethics and specimens
According to the Declaration of Helsinki 1964, this study has been approved by the Health Research Ethics Committee Faculty of Medicine Universitas Indonesia - Cipto Mangunkusumo Hospital (No. 205/UN2.F1/ETIK/2016).
MSC specimens consisting of three ASCs and three UCSCs samples were obtained from HayandraLab and Stem Cell Medical Technology Integrated Service Unit, Cipto Mangunkusumo Central Hospital Faculty of Medicine Universitas Indonesia, Jakarta Indonesia, and have been characterized by the expression of stromal cell markers, i.e. CD73, CD90 positive and CD34 negative, as well as multidifferentiation capabilities to osteogenic, chondrogenic and adipocyte lineages as reported in our previous study26,27.
BCSCs (ALDH+) were obtained from Cell Culture Laboratory for Cancer Stem Cells, Department Biochemistry and Molecular Biology Faculty of Medicine Universitas Indonesia, Jakarta Indonesia. The BCSC specimen has been isolated by ALDEFLUOR™ assay for subsequent downstream assessment (Fluorescence Activated Cell Sorting in Tsukuba University, 2015).
Cell cultures
MSCs were grown in Minimum Essential Medium Alpha (α-MEM) supplemented with 10% FBS, while BCSCs were grown in non-serum DMEM/F12 medium. Both cell cultures were supplemented with 1% penicillin-streptomycin and 1% amphotericin. Cells were incubated under standard conditions (5% CO2 and 37°C). The media were replaced every 3 days and cells were subcultured when confluence was obtained.
Preparation of MSC-conditioned medium (MSC-CM)
Early-passage human MSCs (P3-P5) were grown to 70–80% confluence in α-MEM medium with 10% FBS. Culture medium was removed and the cells were washed three times by PBS 1x to remove any residual serum. The cells were then grown in non-serum α-MEM medium under standard conditions for 24 hours. MSC-CM was centrifuged at 200xg for 10 min to remove cell debris and filtered using 0.22 μm filters. Subsequently, CM was stored at -20°C and used within 3 days. To prepare the CM for each experiment, CM was diluted with DMEM/F12 to obtain a 50% (v/v) concentration.
Incubation of BCSCs (ALDH+) with MSC-CM and viability assay
BCSCs (ALDH+) were harvested and counted using an automatic cell counter (Luna®). About 5 x 105 cells with more than 90% viability were grown in non-serum DMEM/F12 medium. After 24 hours, the BCSC medium was replaced with 50% (v/v) MSCs-CM. After 72 hours of incubation, cells were harvested and counted using Tryphan blue exclusion dye assay. Cells that were grown in 50% (v/v) non-serum α-MEM medium were used as a control. The experiment was performed in triplicate.
RNA isolation and qRT-PCR assay
Isolation of total RNA was performed according to the manufacturer’s instruction (TriPure Isolation Reagent®, Roche). The RNA concentration was measured using a MicroDrop spectrophotometer (Thermo Scientific Skanlt Software for Varioskan™ Flash Multimode Reader). Quantitative reverse transcriptase PCR was performed using SYBR Green and reverse transcriptase enzyme (One-Step qRT-PCR Kit KAPA™SYBR®FAST). The cycling conditions were 5 minutes at 42°C for cDNA synthesis, 5 minutes at 95°C for inactivation of reverse transcriptase enzyme, then 40 cycles consisting of 30 seconds at 95°C for double stranded denaturation and 20 seconds at annealing gene temperature optimized for annealing stage, 20 seconds at 72°C for elongation stage. 18S rRNA was used as an internal control. The normalized fold expression was obtained using the 2-ΔΔCT (Livak) method. Primers used for qRT-PCR were obtained from IDT® (Table 1).
Table 1. Primer sequences used in the qRT-PCR.
| Gene | Primer Forward | Primer Reverse | Amplicon
Length (bp) |
|---|
| ALDH1A3 | 5’-CGA CCT GGA GGG CTG TAT TA-3’ | 5’-TGG TGA AGC ACA CGA CGT T-3’ | 104 |
| TGF-β1 | 5’-GCC TTT CCT GCT TCT CAT GG-3’ | 5’-CTC CGT GGA GCT GAA GCA ATA-3’ | 105 |
| TβRI | 5’- ACT TCC AAC TAC TGG CCC TTT-3’ | 5’-AGA TGC AGA CGA AGC ACA CT-3’ | 100 |
| 18S rRNA | 5’- AAA CGG CTA CCA CAT CCA AG-3’ | 5’-CCT CCA ATG GAT CCT CGT TA-3’ | 155 |
Statistical analysis
All relative gene expression data were analyzed using unpaired Student’s t-test, SPSS 20 and presented as mean ± standard error.
Results
Morphologies of MSCs and BCSCs
BCSCs showed sphere formation within 2–3 days after plating (Figure 1a). UCSCs and ASCs used in this study adhered within hours after plating and displayed spindle-fibroblast-like morphology (Figure 1b–c). Both MSCs gradually fused into a single layer after cell confluence has been reached. Multidifferentiation capability of ASCs and UCSCs have been verified in our previous study26,27.

Figure 1.
Morphologies of BCSCs (a), UCSCs (b), and ASCs (c). About 1x105 cells were plated in each well of a 12-well plate and were grown under standard conditions as described under Materials and Methods. After 2–3 day incubation, cell morphology was observed under inverted microscope at 100x magnification. BCSCs, breast cancer stem cells; UCSCs, umbilical cord-derived stem cells; ASCs, adipose-derived stem cells; CM, conditioned medium.
Expression of ALDH1A3 gene in BCSCs treated with CM of UCSCs and ASCs (Figure 2)
Treatment of UCSC-CM to BCSCs could significantly increase the relative expression of ALDH1A3 gene (1.79 times, p=0.001) compared with the control cells grown in 50% (v/v) non-serum α-MEM medium. In contrast, ASC-CM had no significant effect on ALDH1A3 gene expression in BCSCs (1.18 times, p=0.316) compared with the control cells. Nevertheless, the effect of ASC- was significantly lower than that of UCSC-CM (p=0.024) These results indicate that ALDH1A3 was distinctly expressed in BCSCs treated with UCSC- and ASC-CM.
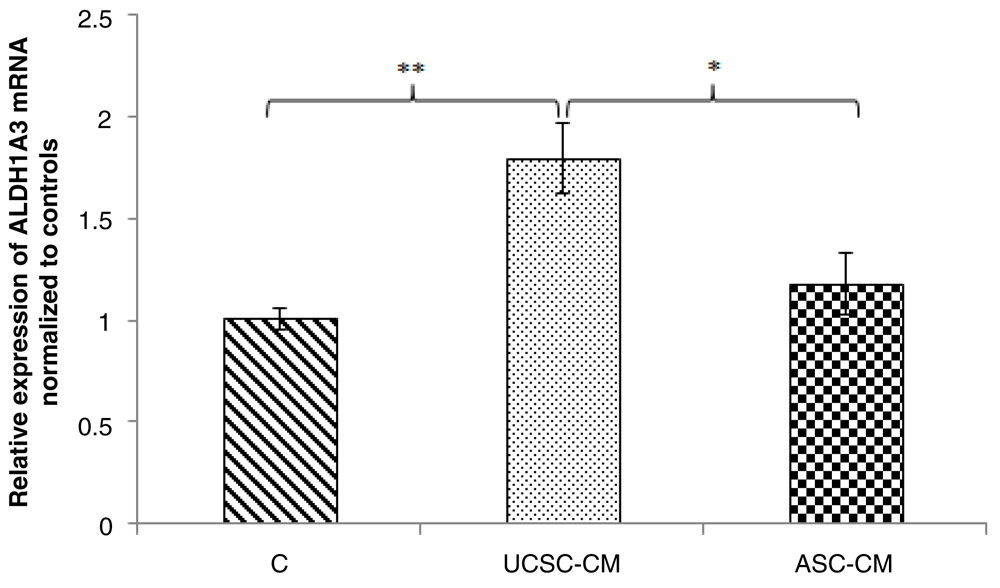
Figure 2.
Relative expression of ALDH1A3 mRNA in BCSCs. Human BCSCs were treated with 50% (v/v) UCSC-CM and ASC-CM, respectively. As a control, BCSCs were treated in 50% (v/v) non-serum α-MEM medium. After 72-hour incubation, total RNA was isolated and quantitative reverse transcriptase PCR was performed to determine ALDH1A3 mRNA expression levels in human BCSCs.The Cq obtained was normalized to 18S rRNA and control cells. Data is presented as mean ± SE. Significance differences are considered at *p<0.05; **p<0.01 (Student’s t-test). BCSCs, breast cancer stem cells; UCSCs, umbilical cord-derived stem cells; ASCs, adipose-derived stem cells; CM, conditioned medium.
| Data for Figure 2 (ALDH1A3 Cq) | | | | | |
|---|
| | | | | |
| ?-MEM (Control) | | UCSC-CM | | ASC-CM | |
| 25.97 | | 25.35 | | 24.89 | |
| 25.94 | | 24.21 | | 26.27 | |
| 25.17 | | 25.02 | | 25.71 | |
| 26.07 | | 25.16 | | 25.83 | |
| 25.6 | | 24.13 | | 25.86 | |
Dataset 2.Dataset 2. Data for Figure 2 (ALDH1A3 Cq value).
http://dx.doi.org/10.5256/f1000research.13609.d194563ALDH1A3 Cq was used to calculate ALDH1A3 mRNA expression levels using Livak formula as demonstrated in Figure 2 (Control: 50% (v/v) a-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells).Expression of TGF-β1 and TβRI genes in BCSCs treated with CM of UCSCs and ASCs (Figure 3)
In attempt to analyze the effect of MSC-CM on TGF-β signaling, we determined the relative mRNA expressions of TGF-β1 and its receptor, TβRI. UCSC-CM significantly increases the relative expression of TGF-β1 (1.72 times, p=0.003) and TβRI (1.54 times, p=0.000). In contrast to this data, ASCs-CM significantly decreases the expression of TGF-β1 (0.64, p=0.003) and TβRI (0.76, p=0.014) in BCSCs compared with controls. Additionally, UCSC- and ASC-CM showed differential effects on either TGF-β1 (p=0.000) or TβRI (p=0.000) expression of BCSCs, suggesting that UCSC and ASC secretomes may be different in content and levels of growth factors, thereby influencing differential regulation of TGF-β1 and TβRI expressions.
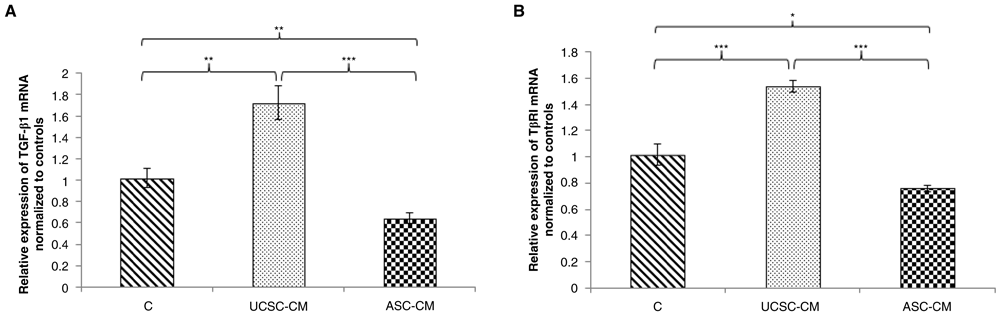
Figure 3. Relative expression of TGF-β1 (A) and TβRI (B) mRNA in BCSCs.
Human BCSCs were treated with 50% (v/v) UCSC-CM and ASC-CM, respectively. As a control, BCSCs were treated in 50% (v/v) non-serum α-MEM medium. After 72-hour incubation, total RNA was isolated and quantitative reverse transcriptase PCR was performed to determine TGF-β1 and TβRI mRNA expression levels in human BCSCs.The Cq obtained was normalized to 18S rRNA and control cells. Data is presented as mean ± SE). Significance differences are considered at *p<0.05; **p<0.01; ***p<0.001 (Student’s t-test). BCSCs, breast cancer stem cells; UCSCs, umbilical cord-derived stem cells; ASCs, adipose-derived stem cells; CM, conditioned medium.
| Data for Figure 3A (TGFb1 Cq) | | | | | |
|---|
| | | | | |
| ?-MEM (Control) | | UCSC-CM | | ASC-CM | |
| 16.3 | | 15.66 | | 17.05 | |
| 16.14 | | 15.13 | | 16.65 | |
| 16.2 | | 15.48 | | 16.71 | |
| 16.47 | | 15.3 | | 16.93 | |
| 16.12 | | 15.68 | | 16.86 | |
Dataset 3.Dataset 3. Data for Figure 3A (TGF-b1 Cq value).
http://dx.doi.org/10.5256/f1000research.13609.d194564TGF-b1 Cq was used to calculate TGF-b1 mRNA expression levels using Livak formula as demonstrated in Figure 3A (Control: 50% (v/v) a-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells).| Data for Figure 3B (TbRI Cq) | | | | | | |
|---|
| | | | | | |
| �-MEM (Control) | | UCSC-CM | | ASC-CM | | |
| 18.98 | | 18.57 | | 19.02 | | |
| 18.85 | | 17.83 | | 19.55 | | |
| 18.68 | | 18.3 | | 19.23 | | |
| 18.98 | | 18.42 | | 19.14 | | |
| 18.58 | | 17.48 | | 19.14 | | |
Dataset 4.Dataset 4. Data for Figure 3B (TbRI Cq value).
http://dx.doi.org/10.5256/f1000research.13609.d194565TbRI Cq was used to calculate TbRI mRNA expression levels using Livak formula as demonstrated in Figure 3B (Control: 50% (v/v) a-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells).| Data for Figure 2, 3A and 3B (18S rRNA Cq) | | | | | |
|---|
| | | | | |
| �-MEM (Control) | | UCSC-CM | | ASC-CM | |
| 11.27 | | 11.41 | | 11.04 | |
| 11.18 | | 10.87 | | 11.31 | |
| 10.54 | | 11.2 | | 11.15 | |
| 11.74 | | 11.32 | | 11.18 | |
| 10.9 | | 10.6 | | 11.2 | |
Dataset 5.Dataset 5. Data for Figures 2, 3A, and 3B (18S rRNA Cq value).
http://dx.doi.org/10.5256/f1000research.13609.d19456618S rRNA Cq was used to calculate ALDH1A3, TGF-b1, and TbRI mRNA expression levels using Livak formula (Control: 50% (v/v) a-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells).Discussion
Recently, the utilization of secretomes contained within MSC-CM has begun to be performed on various rejuvenation therapies. However, the possible consequences that may arise due to the interaction between biologically active factors (secretomes) and cancer cells has not yet been elucidated. As with MSCs, their secretomes have also been reported to either suppress or promote growth and development of cancer3. These contradictory effects may be due to different composition of secretomes, with one of the causes being the fact that they are obtained from different MSC sources4,28.
In this study, we found that secretomes within UCSC-CM have a higher ability to promote ALDH1A3 gene expression in BCSCs, indicating the higher paracrine signaling activity of UCSC secretomes when compared to those of ASCs (Figure 2). This result is in contrast with the effect of MSC secretomes on ALDH1A1 expression of BCSCs demonstrated in our previous study, which revealed that ALDH1A1 mRNA expression was significantly reduced by UCSC-CM and increased by ASC-CM26. In that study, we also found that ALDH1A1 expression is in line with the expression of OCT-4 and SOX2. Therefore, we suggest that unlike ALDH1A1, ALDH1A3 does not prominently contribute to the pluripotency of BCSCs. This has been verified by in silico analysis that showed that there is no direct interaction between ALDH1A3 and pluripotency markers, OCT4, SOX2, NANOG, and KLF4 (in press; Wanandi SI, Purnamawati, Tamara A, Putri KT, Simadibrata D). That study has also indicated that ALDH1A1 expression levels in MSCs were more similar to OCT4 rather than to ALDH1A3 levels, suggesting the role of ALDH1A1 on pluripotency.
In the present study, we highlighted that the effects of UCSC and ASC secretomes on ALDH1A3 were consistent with the expressions of TGF-β1 and its receptor, TβRI, in BCSCs (Figure 3). In spite of that, the effects of UCSC on ALDH1A3, TGF-β1, and TβRI expressions were opposite to those of ASC secretomes, in which UCSC up-regulated, while ASC secretomes down-regulated those gene expressions. Previous studies have reported that different MSC sources have different growth factor contents and levels4,28. Very recently, we have also demonstrated that UCSCs and ASCs expressed different levels of either ALDH1A1 or ALDH1A3 (in press; <Wanandi SI, Purnamawati, Tamara A, Putri KT, Simadibrata D>). These two isozymes of ALDH1 have newly been confirmed to have differential functional roles in facilitating aggressiveness of human breast cancer cells29. ALDH1A1 suppresses proliferation, metastatic properties and therapy resistance of breast cancer cells, whereas ALDH1A3 has predominant effect on ALDH activity. These results supported our previous study that presented the increase of viability and pluripotency in ASC secretomes-treated BCSCs in line with the increase of ALDH1A126.
Moreover, the current study also underlines the effect of UCSC and ASC secretomes on TGF-β1 autocrine signaling in BCSCs, as revealed by TGF-β1 and its receptor, TβRI expressions (Figures 3A and B). We suggest that secretomes of UCSCs and ASCs presumably contained growth factors including TGF-β1 that could auto-induce TGF-β1 signaling in BCSCs. Due to TGF-β1 paradox, tumor proliferation could be stimulated or inhibited either via differential ERK depending on relative level of TGF-β1 present in tumor microenvironment25. A plausible explanation of TGF-β1 autocrine signaling induction in our BCSCs is as a cellular homeostasis against reduced cell viability and pluripotency due to enhanced ALDH1A3 and diminished ALDH1A1 expression after supplementation with UCSC secretomes26,28.
In conclusion, the differential effects of UCSC and ASC secretomes on ALDH1A3 expression in human BCSCs may be related to autocrine TGF-β1 signaling, as opposed to ALDH1A1 which regulates BCSC viability and pluripotency. In depth studies are further required to elaborate signaling factors within UCSC and ASC secretomes that specifically regulate ALDH1A1 and ALDH1A3 expressions in relation to TGF-β1 autocrine signaling and its impact on the aggressiveness of BCSCs.
Data availability
Dataset 1: Raw unedited images for Figure 1A, 1B, and 1C. DOI, 10.5256/f1000research.13609.d19456230
Dataset 2: Data for Figure 2 (ALDH1A3 Cq value). ALDH1A3 Cq was used to calculate ALDH1A3 mRNA expression levels using Livak formula as demonstrated in Figure 2 (Control: 50% (v/v) α-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells). DOI, 10.5256/f1000research.13609.d19456331
Dataset 3: Data for Figure 3A (TGF-β1 Cq value). TGF-β1 Cq was used to calculate TGF-β1 mRNA expression levels using Livak formula as demonstrated in Figure 3A (Control: 50% (v/v) α-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells). DOI, 10.5256/f1000research.13609.d19456432
Dataset 4: Data for Figure 3B (TβRI Cq value). TβRICq was used to calculate TβRI mRNA expression levels using Livak formula as demonstrated in Figure 3B (Control: 50% (v/v) α-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells). DOI, 10.5256/f1000research.13609.d19456533
Dataset 5: Data for Figure 2, Figure 3A, and Figure 3B (18S rRNA Cq value). 18S rRNA Cq was used to calculate ALDH1A3, TGF-β1, and TβRI mRNA expression levels using Livak formula (Control: 50% (v/v) α-MEM-treated cells; UCSC-CM: conditioned medium of umbilical cord-derived stem cells; ASC-CM: conditioned medium of adipose-derived stem cells). DOI, 10.5256/f1000research.13609.d19456634
Competing interests
No competing interests were disclosed.
Grant information
Publication of this work was supported by the grant of International Indexed Publication for Final Assignment of the Postgraduate Student from Universitas Indonesia Year 2017 (557/UN2.R3.1/HKP.05.00/2017).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We thank Dr. dr. Novi Silvia Hardiany, M.Biomed (Dept of Biochemistry and Molecular Biology FKUI) dr.Isabella Kurnia Liem, M.Biomed., PA., Ph.D (Cell Medical Technology Integrated Service Unit, RSCM-FKUI), dr. Karina, SpBP-RE (HayandraLab) and Dr. dr. Reza Y. Purwoko, SpKK (Erpour Laboratory) for their generosity in providing BCSCs (ALDH+), USCSc, and ASCs, respectively.
Faculty Opinions recommendedReferences
- 1.
Madrigal M, Rao KS, Riordan NH:
A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods.
J Transl Med.
2014; 12(1): 260.PubMed Abstract
| Publisher Full Text
| Free Full Text
- 2.
Hu L, Hu J, Zhao J, et al.:
Side-by-side comparison of the biological characteristics of human umbilical cord and adipose tissue-derived mesenchymal stem cells.
Biomed Res Int.
2013; 1–12. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Zimmerlin L, Park TS, Zambidis ET, et al.:
Mesenchymal stem cell secretome and regenerative therapy after cancer.
Biochimie.
2013; 95(12): 2235–45. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 4.
Zhu W, Huang L, Li Y, et al.:
Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth.
Cell Cycle.
2011; 10(18): 3198–207. PubMed Abstract
| Publisher Full Text
- 5.
Ohta N, Ishiguro S, Kawabata A, et al.:
Human umbilical cord matrix mesenchymal stem cells suppress the growth of breast cancer by expression of tumor suppressor genes.
PLoS One.
10(5): e0123756. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 6.
Doi C, Maurya DK, Pyle MM, et al.:
Cytotherapy with naive rat umbilical cord matrix stem cells significantly attenuates growth of murine pancreatic cancer cells and increases survival in syngeneic mice.
Cytotherapy.
2010; 12(3): 408–17. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Rhee KJ, Lee JI, Eom YW:
Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression.
Int J Mol Sci.
2015; 16(12): 30015–33. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Kudo-Saito C:
Cancer-associated mesenchymal stem cells aggravate tumor progression.
Front Cell Dev Biol.
2015; 3: 23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Yagi H, Kitagawa Y:
The role of mesenchymal stem cells in cancer development.
Front Genet.
2013; 4: 261. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 10.
Pawitan JA:
Prospect of stem cell conditioned medium in regenerative medicine.
Biomed Res Int.
2014; 2014(2014): 1–14 965849. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Tran C, Damaser MS:
Stem cells as drug delivery methods: application of stem cell secretome for regeneration.
Adv Drug Deliv Rev.
2015; 82–83: 1–11. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Guan J, Chen J:
Mesenchymal stem cells in the tumor microenvironment.
Biomed Rep.
2013; 1(4): 517–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Rodriguez-Torres M, Allan AL:
Aldehyde dehydrogenase as a marker and functional mediator of metastasis in solid tumors.
Clin Exp Metastasis.
2016; 33(1): 97–113. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Shima H, Yamada A, Ishikawa T, et al.:
Are breast cancer stem cells the key to resolving clinical issues in breast cancer therapy?
Gland Surg.
2017; 6(1): 82–88. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Bednarz-Knoll N, Nastały P, Żaczek A, et al.:
Stromal expression of ALDH1 in human breast carcinomas indicates reduced tumor progression.
Oncotarget.
2015; 6(29): 26789–803. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Tomita H, Tanaka K, Tanaka T, et al.:
Aldehyde dehydrogenase 1A1 in stem cells and cancer.
Oncotarget.
2016; 7(10): 11018–32. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 17.
Allahverdiyev AM, Bagirova M, Oztel ON, et al.:
Aldehyde Dehydrogenase: Cancer and Stem Cells.
InTech. [Internet] . 2012. [cited 2017 Jul 29]; Publisher Full Text
- 18.
Khoury T, Ademuyiwa FO, Chandraseekhar R, et al.:
Aldehyde dehydrogenase 1A1 expression in breast cancer is associated with stage, triple negativity, and outcome to neoadjuvant chemotherapy.
Mod Pathol.
2012; 25(3): 388–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Zhao D, Mo Y, Li MT, et al.:
NOTCH-induced aldehyde dehydrogenase 1A1 deacetylation promotes breast cancer stem cells.
J Clin Invest.
2014; 124(12): 5453–65. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Marcato P, Dean CA, Pan D, et al.:
Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis.
Stem Cells.
2011; 29(1): 32–45. PubMed Abstract
| Publisher Full Text
- 21.
Raha D, Wilson TR, Peng J, et al.:
The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation.
Cancer Res.
2014; 74(13): 3579–90. PubMed Abstract
| Publisher Full Text
- 22.
Marcato P, Dean CA, Liu RZ, et al.:
Aldehyde dehydrogenase 1A3 influences breast cancer progression via differential retinoic acid signaling.
Mol Oncol.
2015; 9(1): 17–31. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Lebrun JJ:
The Dual Role of TGFβ in Human Cancer: From Tumor Suppression to Cancer Metastasis.
ISRN Mol Biol.
2012; 2012: 381428. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Principe DR, Doll JA, Bauer J, et al.:
TGF-β: Duality of function between tumor prevention and carcinogenesis.
J Natl Cancer Inst.
2014; 106(2): djt369. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Zhang Q, Yu N, Lee C:
Mysteries of TGF-β Paradox in Benign and Malignant Cells.
Front Oncol.
2014; 4: 94. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Purnamawati P, Pawitan JA, Rachman A:
Secretomes of Adipose and Umbilical Cord-Derived Stem Cells Affect ALDH1A1 Expression in Breast Cancer Stem Cells.
Adv Sci Lett.
2017; 23(7): 6701–04. Publisher Full Text
- 27.
Pawitan JA, Kispa T, Mediana D, et al.:
Simple production method of umbilical cord derived mesenchymal stem cell using xeno-free materials for translational research.
J Chem Pharm Res.
2015; 7(8): 652–6. Reference Source
- 28.
Heo JS, Choi Y, Kim HS, et al.:
Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue.
Int J Mol Med.
2016; 37(1): 115–25. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Croker AK, Rodriguez-Torres M, Xia Y, et al.:
Differential Functional Roles of ALDH1A1 and ALDH1A3 in Mediating Metastatic Behavior and Therapy Resistance of Human Breast Cancer Cells.
Int J Mol Sci.
2017; 18(10): pii: E2039. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Purnamawati P, Pawitan JA, Rachman A, et al.:
Dataset 1 in: Effects of umbilical cord- and adipose-derived stem cell secretomes on ALDH1A3 expression and autocrine TGF-β1 signaling in human breast cancer stem cells.
F1000Research.
2018. Data Source
- 31.
Purnamawati P, Pawitan JA, Rachman A, et al.:
Dataset 2 in: Effects of umbilical cord- and adipose-derived stem cell secretomes on ALDH1A3 expression and autocrine TGF-β1 signaling in human breast cancer stem cells.
F1000Research.
2018. Data Source
- 32.
Purnamawati P, Pawitan JA, Rachman A, et al.:
Dataset 3 in: Effects of umbilical cord- and adipose-derived stem cell secretomes on ALDH1A3 expression and autocrine TGF-β1 signaling in human breast cancer stem cells.
F1000Research.
2018. Data Source
- 33.
Purnamawati P, Pawitan JA, Rachman A, et al.:
Dataset 4 in: Effects of umbilical cord- and adipose-derived stem cell secretomes on ALDH1A3 expression and autocrine TGF-β1 signaling in human breast cancer stem cells.
F1000Research.
2018. Data Source
- 34.
Purnamawati P, Pawitan JA, Rachman A, et al.:
Dataset 5 in: Effects of umbilical cord- and adipose-derived stem cell secretomes on ALDH1A3 expression and autocrine TGF-β1 signaling in human breast cancer stem cells.
F1000Research.
2018. Data Source
Comments on this article Comments (0)