Keywords
nanocapsules, Eudragit RL 100, Glioma, Piper nigrum
This article is included in the Nanoscience & Nanotechnology gateway.
nanocapsules, Eudragit RL 100, Glioma, Piper nigrum
We added some more explanation in the method session of the revised manuscript about how the drug-loaded nanocapsules were formed, the sample preparation and the detail on SEM analysis (magnification at 600x). As for the preparation of nanocapsules, the DMEO can be encapsulated into nanocapsules at a maximum concentration of 1 mg/ml with the ratio maintained at the 1:40; 1:45; and 1:50 drug/polymer ratio. We replaced "polymerized nanocapsules" with "polymeric nanocapsules". Meanwhile, we highlighted that the PXRD data aimed to characterize the crystallinity of DMEO in the formulated nanocapsules.
See the authors' detailed response to the review by Rani Sauriasari
See the authors' detailed response to the review by Heni Rachmawati
The hedgehog (Hh) pathway is required for the growth and proliferation of various cancers1. The signaling begins with the binding of Hh protein ligand to its membrane receptor Ptch, which represses the activity of Smoothened (Smo). Smo in turn promotes the expression of the GLI (Glioma-associated oncogene) family of transcription factors, leading to tumor development2. The direct association of GLI with a specific binding site (5’-GACCACCCA-3’) in the promoter region of the target gene has been reported to contribute to pancreatic and prostate cancer cells3. We previously reported Hedgehog/GLI inhibitors from various plants4–6. We also isolated five lignans ((8R*,8’R*)-9-hydroxy-3,4-dimethoxy-3’,4’-methylenedioxy-9,9’-epoxylignan, kusunokinin, haplomyrfolol, dihydroclusin) including a new epoxylignan dmeo from Piper nigrum using the immobilization of GLI-GST on carboxylic acid magnetic dynabeads. DMEO was confirmed to have Hh signaling inhibitory activity and to be selectively cytotoxic against PANC1. Meanwhile the synthetic epoxylignan of DMEO related compound was reported to inhibit the mRNA expression of protein patched homolog (Ptch) in human pancreatic cancer cells (PANC1) and thus is considered to be a prospective drug candidate to treat cancer related to the GLI signaling pathway. However, poor solubility remains the main limitation of DMEO7, hence making drug administration in vivo difficult. The nanoencapsulation of DMEO is one of the ways of overcoming the problem.
Nanoencapsulation techniques are particularly important to protect drugs from degradation in biological fluids and improve their penetration into cells. The techniques are also beneficial for hydrophobic molecules because the ultra-dispersed pharmaceutical dosage forms that nanoencapsulation provides allow rapid drug dissolution8. The nanoencapsulation of DMEO within a suitable polymer is considered to be a good way to ameliorate its poor solubility, because the polymer acts as a rate-controlling membrane to obtain the desired controlled release. The physical stability of pure compounds remains the greatest challenge for pharmaceutical scientists seeking to exploit higher solubility properties. A gold standard revealed by the International Council for Harmonization (ICH) stated that physical stability tests of compounds should be performed within accelerated (6 months) and/or long-term (12 months) storage conditions9. The physical stability was examined using powder X-ray diffraction (PXRD) analysis.
The objective of this study was to characterize the physical stability of DMEO-loaded nanocapsules, which were optimized by varying the polymer concentration to obtain stable spherical particles. The most stable particles would result from the best concentration ratio between polymers and stabilizers, ultimately improving the dissolution rate of poorly water soluble drugs.
Eudragrit RL 100 and polyvinyl alcohol were purchased from Sigma-Aldrich Ltd. (St Louis, MO, USA). DMEO was obtained from the Pharmaceutical Chemistry Laboratory of Hasanuddin University (Indonesia). Methanol, ethyl acetate, acetonitrile, chlorophorm and demineralized water were purchased from Merck, Indonesia. All chemicals and solvents were of analytical or pharmaceutical grade.
Nanocapsules were prepared by an emulsion-diffusion method using Eudragit RL (ERL, Merck Ltd) at various concentrations (1%, 1.5% and 2%). Briefly, the ERL polymer (100, 150 and 200 mg) were dissolved respectively in 10 mL of ethyl acetate saturated with water. Each of this organic phase was then emulsified with 40 mL of aqueous phase, saturated with ethyl acetate, containing 300 mg of Polyvinyl alcohol (PVA) using a high speed homogenizer (ultra-turax T 25, Germany) at 1500 rpm for 60 minutes. Deionized water (150 mL) was then added to the emulsion to induce the diffusion of ethyl acetate into the continuous phase leading to the formation of nanocapsules. The organic solvent and the water phase were evaporated under reduced pressure to obtain a concentrated suspension of 40 mL.
DMEO was previously synthesized and characterized as a white powder10. 10 mg of DMEO was dissolved in 10 mL of methanol, which was then added to polymeric nanocapsules. The DMEO can be encapsulated into nanocapsules at a maximum concentration of 1 mg/ml with the ratio maintained at the 1:40; 1:45; and 1:50 drug/polymer ratio. DMEO-loaded nanocapsules were dried in a desiccator until constant weight. They were then kept in a closed glass vial and stored at 25°C.
The size of DMEO-loaded nanocapsules was analyzed by laser diffraction using a Partica LA-950 laser diffraction particle size analyzer (Horiba Ltd, Japan). Dried particles (5 mg) were dispersed in Miglylol 812 using a UP50H ultrasound processor (Hielscher, Germany) and analyzed in triplicate. The surface characteristics of the particles were observed by scanning electron microscopy (SEM) (Jeol, JSM-5600 LV, Japan) magnification at 600x. The yields of the particles were calculated by the sum of the weights of all components, discounting the content of water in the suspensions. After stirring the powders in acetonitrile for 90 minutes at room temperature followed by centrifugation and filtration (GVWP membrane, 0.45 µm, Millipore), the nanoencapsulation efficiency of DMEO was determined by UV-Vis spectroscopy (Shimadzu; 229 nm absorbance detector). The nanoencapsulation efficiency (%) of the powder was calculated from the correlation of the theoretical and experimental DMEO concentration. The onset of its crystallization was examined before and after 6 months of storage at 25°C, using an X-Ray Diffractometer (XRD-7000, SHIMADZU) with Cu K α radiation (λ = 0.154 nm). The diffraction patterns were analyzed using X’Pert Highscore Plus (version 2.2d).
SEM analysis reveals that all DMEO-loaded nanocapsules are mostly spherical (Figure 1). As the polymer content increased, the encapsulation efficiency and mean particle size also increased, as seen in Table 1, suggesting that nanoencapsulation minimized deactivation of the drugs during the delivery process due to the protection from the polymer shell. This might ensure sufficient amounts of drug reaching the targeted areas. ERL is a copolymer of partial esters of acrylic acid containing low amounts of a quaternary ammonium group. ERL is a water-soluble polymer that plays a crucial role in controlling drug release because its water uptake characteristic contributes to the swelling of the polymers.
| Sample Code | DMEO (mg) | Polymer (mg) | Mean Particle Size (nm) | Encapsulation Efficiency (%) | |
|---|---|---|---|---|---|
| PVA | ERL | ||||
| F1 | 10 | 300 | 100 | 227.55±0.231 | 89.53±0.307 |
| F2 | 10 | 300 | 150 | 253.96±0.012 | 89.46±0.211 |
| F3 | 10 | 300 | 200 | 255.09±0.455 | 90.31±0.352 |
In crystalline materials, atoms are periodically arranged, but in non-crystalline materials, atoms are randomly arranged. Figure 2 shows that the peaks of DMEO do not possess that periodicity, while the characteristic peak falls at 19.3° 2θ. After the physical mixtures were subjected to various concentrations of polymers and stabilizers, all the peaks were re-observed at 0 and 6 months of storage. No crystalline peaks were observed in the diffraction patterns, as shown in Figure 2a, 2b and 2c (upper, down), suggesting that the particles remain spherical throughout the duration of the stability study. The unchanged position of the largest peak of DMEO, which remains at 19.3° 2θ (Figure 2a, 2b and 2c (upper, down)), indicates that there is no change in the diffraction pattern of DMEO after 6 months of storage. It is important for the DMEO particles to remain stable during 6 months of storage to ensure that the resulting DMEO-loaded nanocapsules meet the minimal standard requirements of drug formulation stability.
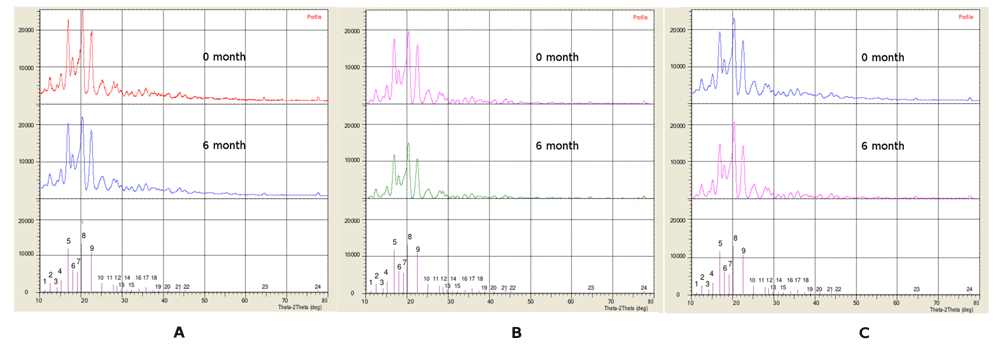
(A) X-ray diffraction patterns of DMEO-loaded nanocapsules of F1 after 0 and 6 months of storage (B) X-ray diffraction patterns of DMEO-loaded nanocapsules of F2 after 0 and 6 months of storage (C) X-ray diffraction patterns of DMEO-loaded nanocapsules of F3 after 0 and 6 months of storage.
The polymer-based encapsulation may be beneficial regarding improved short-term physical stability. The nanoencapsulation of DMEO yielded entrapment efficiencies of 89.53±0.307, 89.46±0.211, 90.31±0.352% with particle sizes of 227.55±0.231, 253.96±0.012, and 255.09±0.455 nm, respectively. It is generally considered that higher molecular weight polymers have less entropy loss related to their fast motion, which results in a higher affinity to drug surfaces10, and they therefore perhaps have better steric formation. The charge along the polymer chains can build a strong double layer surrounding the particles, granting electrostatic stabilization and therefore smaller particle size11.
The results showed that the polymer concentration influenced the particle size and entrapment efficiency. The 1% polymer concentration formula (F1) fulfilled the requirement of stable nanocapsules, including spherical and uniform surface morphology. This formula exhibited the greatest percentage of nanocapsules’ weight (10.24%) but had the smallest particle size (227.55 nm). Meanwhile, F3 showed the greatest entrapment efficiency (90.31%). We chose F1, F2 and F3 for the further physical stability study using XRD to see whether the different concentrations of polymer interfered with the stability of DMEO in nanocapsules. Polymers were expected to adsorb to the surface of the nanocapsules, providing both electrostatic and steric repulsion among particles, therefore producing nanoparticles with decreased particle sizes and improved physical stability. The polymers may adsorb to the drug surfaces through several points along the polymer chains, enabling the loops and tails of the polymers to extend into the liquid medium, providing a steric effect.
The key finding in this work was that higher concentrations of polymer in the stirring solution during the production process yielded higher encapsulation efficiencies and smaller particle sizes. Tuning the polymer ratio was essential to obtaining spherical particles, without necessarily affecting the stability of the obtained nanocapsules.
Dataset 1: Raw data for particle size, particle yields, nanoencapsulation efficiencies and X-ray diffraction pattern values. 10.5256/f1000research.13047.d19534812
This work was supported by the Ministry of Research, Technology and Higher Education of Indonesia through a grant from WCU-UNHAS 2016-2017.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful to Dahlan Tahir from the Department of Physics, Faculty of Life Sciences for providing necessary research facilities.
Supplementary File 1: Structure of 3’,6-dimethoxy-3’’,4’’-(methylenedioxy)-2,5-epoxylignan-4’-ol (DMEO). 1H and 13C NMR Data of DMEO
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmaceutics with focusing on nanotechnology-based drug formulation and delivery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 30 Jul 18 |
read | |
|
Version 1 01 Mar 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)