Keywords
Tuberculosis, latent tuberculosis infection, human cytomegalovirus, epidemiology, age pattern, risk factor
This article is included in the World TB Day collection.
Tuberculosis, latent tuberculosis infection, human cytomegalovirus, epidemiology, age pattern, risk factor
With 10.4 million new cases and 1.7 million deaths per year, tuberculosis (TB) remains a major global health problem1. Only 5%–15% of individuals infected with Mycobacterium tuberculosis (Mtb) ever develop TB disease, and over 50% of these do so within two years after infection2. Although risk factors for progression to TB disease have been identified3, disease occurrence cannot be accurately predicted4.
Recent data suggest that infection with human cytomegalovirus (HCMV) is a predictor of TB disease in infants. In a cohort study of South African infants, an HCMV-specific IFN-γ T-cell response was associated with a 2.2-fold increased risk of TB disease over a period of up to 3 years. A similar response to Epstein-Barr virus (EBV) showed no such associations5. HCMV-positive and HCMV-negative infants had distinct immune pathways associated with TB disease. Although CD8+ T-cell activation was a distinguishing feature of HCMV-positive infants, the proposed immunological mechanism was impairment of the natural killer (NK) cell response. In African infants, HCMV infection induced profound CD8+ T-cell and NK cell differentiation and poor physical growth5–7.
The possibility that this association between HCMV and TB disease progression is causal, also holds in adults, and thus merits further study is dependent on its epidemiological plausibility. Only few published studies have investigated epidemiological associations between the two diseases8–10. Despite this paucity of direct evidence we argue that the epidemiology of TB and HCMV share important similarities that make HCMV infection a plausible candidate as a cause of TB disease progression.
Various etiological frameworks for TB disease progression have been developed. One proposed by Comstock considers TB disease the result of two hits or causes, one of which is Mtb infection, and the other (still) unknown11. In this framework, factors that strongly increase the risk of TB disease such as HIV infection or anti-tumour necrosis alpha therapy may act as a second hit but would not account for all or most TB cases.
Several factors have been identified that increase the risk of disease progression, such as low body-mass index12, diabetes13, tobacco smoking14, and alcohol abuse15. As their effects are modest another framework has emerged that these are predisposing conditions for disease progression while other, yet unidentified precipitating events are needed to trigger progression to active disease16. Among the precipitating events suggested are respiratory viral infections, notably influenza A. Influenza A induces type I interferons that impair immunity against mycobacteria17. Also, notification of TB tends to peak in the months after winter when most respiratory viruses circulate18, and TB mortality has shown increases during influenza epidemics19,20. However, careful analysis of seasonality data suggests that it is TB transmission rather than disease progression that is increased in winter21. HCMV has been suggested in three studies from Nigeria, Russia and Uganda that all found higher prevalence or levels of IgG HCMV antibodies in diagnosed TB patients compared to healthy controls and patients diagnosed with other diseases8–10.
HCMV, human herpesvirus 5, is a double-stranded DNA virus. After primary infection, usually through mucosal contact, HCMV remains dormant in the host’s myeloid tissues but can reactivate if immunity is compromised. Primary infection is often asymptomatic but can present as mononucleosis with fever, pharyngo-tonsillitis and lymphadenopathy. In congenitally infected infants HCMV may cause severe generalized infection with high case fatality and neurologic sequelae22. Generalized infection also occurs in severely immunocompromised adults, usually through reactivation. During primary infection and reactivation virus is shed in the urine, saliva, breast milk, cervical fluid and semen23. Common routes of transmission are from mother to child during delivery, between children and by sexual contact. Transmission through blood transfusion and solid organ transplantations also occurs.
HCMV viruses show genomic diversity, in particular in genes coding for envelope glycoproteins, and polymorphisms in these genes have been used to genotype strains24,25. Both immunocompromised and immunocompetent individuals can be re-infected and harbour multiple HCMV strains26–28.
Primary HCMV infection is characterized by profound expansion of antigen specific CD8+ and CD4+ T cells and NK cell populations with specificity for HCMV29. HCMV expanded NK cells can display inappropriate homing to tissue infected with other pathogens and lower IFN-γ secretion in response to pathogens30. HCMV has multiple immune evasion strategies29, which may make the microenvironment around latently infected myeloid cells suppressive to T-cell function, potentially creating an environment permissive for mycobacterial growth31.
The lung is a reservoir of HCMV infection and frequently the site of viral reactivation, which drives inflammation and in mice may cause pulmonary fibrosis32. It is therefore possible that replication of Mtb in the lungs could lead to reactivation of latent HCMV or that reactivation of latent HCMV could precipitate progression to TB disease.
Both Mtb and HCMV infections are ubiquitous1,33, and during millions of years of co-evolution have become highly human host-specific34–36. An animal reservoir has been described for neither Mtb nor HCMV (several monkey and rodent species have their distinct CMV species), implying that their epidemiological patterns are entirely determined by transmission between, and carriage by, humans.
We hypothesize that immunologically active HCMV infection, whether primary, reactivation or re-infection, acts as (depending on one’s preferred framework) second-hit or precipitating factor for progression of latent TB infection to TB disease at an epidemiologically relevant scale. We base this on two arguments: their striking similarity in age distribution, and the existence of congruent risk factors [Box 1].
We systematically searched PubMed for the following combinations of keywords: tuberculosis and cytomegalovirus; cytomegalovirus and prevalence or seroprevalence; cytomegalovirus and age; cytomegalovirus and reinfection; cytomegalovirus and sexual; tuberculosis and sexual; tuberculosis and sexual transmitted infections or Chlamydia or gonorrhoea or human papillomavirus; tuberculosis and blood transfusion; cytomegalovirus and blood transfusion; tuberculosis and gastrectomy; cytomegalovirus and renal dialysis; tuberculosis and renal dialysis; tuberculosis and organ transplantation; cytomegalovirus and organ transplantation.
We in addition made use of an extensive review of the literature on age-sex distribution of tuberculosis incidence published by Nagelkerke (2012)37.
The probability of progressing from TB infection to disease has a highly typical age distribution. The classical description of this age pattern is by Comstock et al, who followed 82,269 Puerto Rican children reacting to tuberculin enrolled in 1949–1951 for 8 to 20 years [Figure 1]38. This pattern, confirmed in a systematic review of studies from the pre-chemotherapy era39, is defined by a peak in the first 1–4 years of life, followed by a trough until early puberty, rising to a second peak around the age of 20 years. Analyses of notification and prevalence data from high-incidence countries show that incidence starts to rise again from the sixth decade40,41. Although several explanations for this age pattern have been suggested, none has been proven.
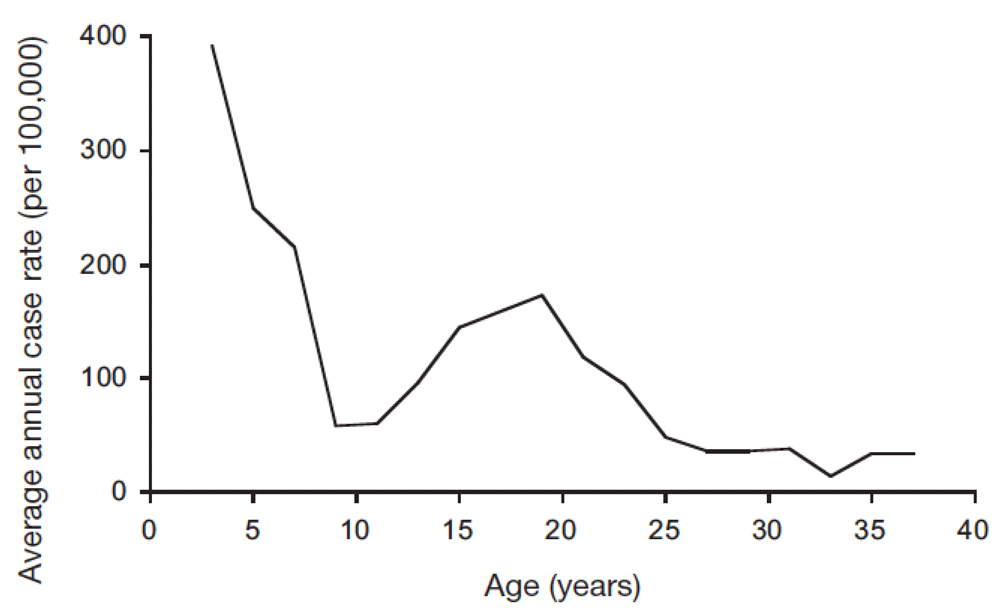
Average annual rate of tuberculosis disease in a cohort of 82,269 Puerto Rican children with a positive tuberculin skin test, by age of disease occurrence. Children were enrolled in the period 1949–1951, and followed for 8 to 20 years. Figure reproduced with permission from Comstock et al. (1974)38.
Infants. Studies from the pre-chemotherapy era showed that, while the risk of infection with Mtb in the first year of life was over 10-fold lower than later in childhood, the risk of progression to disease once infected was much higher with up to 50% of infected infants developing disease39. These high progression rates have been attributed to age-specific maturation of immune responses42, although the mechanisms responsible for this vulnerability have not been elucidated43.
HCMV infection in infants is common44. Depending on the country and socio-economic status of the mother, between 10 and 60% of children are HCMV IgG seropositive (reflecting current or past active infection) by the age of 12–36 months45–52. Important causes are congenital infection and transmission through breastfeeding; >85% of HCMV seropositive women excrete virus in the breastmilk44,53–56. Infants infected through breastfeeding do not develop disease, probably due to protection by maternal antibodies, but do shed virus in saliva and urine intermittently for months, by which they may transmit HCMV to other children and caregivers23,44,48,57. Shedding of HCMV shows a steep decline by the age of 5 years23, coinciding with the age at which TB incidences drop38,39.
Adolescents. The rate of progression to TB disease then remains low until puberty. Several studies have observed an increase in TB incidence from this age onward among children who were exposed to infectious TB patients or had a positive tuberculin response, leading to a peak in incidence in the first half of the third decade38,58–63. This phenomenon has been attributed to hormonal changes, but again without a putative mechanistic pathway64.
Most population-based studies of HCMV seroprevalence show exactly this age pattern: a slow increase in HCMV IgG seroprevalence up to the age of 10–15 years, followed by an acceleration during adolescence45,47–50,52,65–72. One explanation for this increase in seroprevalence is sexual transmission. Various studies found that HCMV conversion among women was associated with sexual activity73–77. However, as several studies of adolescents found no association of HCMV seroprevalence with sexual exposure67,78,79, other transmission routes such as mouth-to-mouth kissing may also be important.
Another indication that HCMV infection may be implicated is the sex difference in TB disease progression in the second decade. For girls the increase in TB incidence starts 2–4 years earlier than for boys, and progression rates tend to remain higher in women than in men for the subsequent two decades, a pattern that was observed before the HIV era in various populations38,59,60,62,63,80–83. This pattern is again reflected in that of HCMV infection. The acceleration of HCMV seroprevalence during puberty and adolescence is steeper in girls than in boys and is higher in women of childbearing age than in men in populations with relatively low HCMV seroprevalence33,48,49,68,84–86. Age-adjusted HCMV seroprevalence does not differ between men and women in populations with high seroprevalence33,87. This may be because IgG seroprevalence measures cumulative infection experience and thus ignores reinfection. HCMV reinfection, identified by DNA typing or strain-specific antibody responses, is a common occurrence in sexually exposed women88–90.
Elderly. Although there is little data on TB progression rates in the elderly, age patterns of TB notifications suggest increased progression rates from the sixth decade onward1,40. In populations with declining incidence rates over the past decades this is partially a cohort effect, whereby younger generations have lower prevalence of latent infection91,92. However in high-incidence countries with little change in TB incidence, notification rates clearly increase at older age1. This is also observed for TB prevalence in population surveys, suggesting that this is not explained by better access to diagnosis1. HCMV infection has been implicated as a cause of age-related decrease in naïve T cells and increase in memory T cells known as immunosenescence93. However, reactivation of HCMV infection is also common at old age, probably reflecting weakening immune control29. Detection of viral DNA increases after the age of 60–70 years94,95, and viral DNA is frequently detected in urine and plasma of elderly people96,97.
Our hypothesis predicts that factors that drive CMV (re-)infection are also risk factors for TB. We highlight the four most important: socio-economic status, sexual contact, blood transfusion, and solid organ transplantation.
Socio-economic status. Incidence and prevalence of CMV infection are associated with poor socio-economic status (SES), between countries as well as within countries and communities33,48,49,84,98–100. This includes association with crowding, in particular the number of young children in household101–103. Several studies found ethnicity or migrant status to be independently associated with age-adjusted CMV prevalence49,68,104, which may partly reflect higher background infection rates in the country of origin. In a US study the association with ethnicity was explained by differences in exposure to infants and sexual risk77.
Also the incidence of TB, often regarded as the archetypal poverty disease, shows a remarkable inverse gradient with SES at the household, regional and country level105–107. This association has been explained mainly by crowding in ill-ventilated spaces conducive to Mtb transmission108, poor nutritional status12,109, alcohol abuse15 and, possibly, indoor air pollution110. Similarly, in low-incidence countries, TB incidences are higher in particular ethnic groups and immigrants111,112, which also may reflect socio-economic disparities and differences in background infection rates113. Very few studies have attempted to investigate whether these and other known risk factors explain all of the observed variation in SES-related TB incidence114.
Sexual contact. The risk of CMV (re-)infection in adults is correlated with measures of sexual activity such as age at first intercourse, recent and lifetime number of sexual partners and condom use, as well as with prevalence of other sexually transmitted infections73,74,76,115–117. Historically, TB has also been associated with sexual promiscuity in medical and popular literature (reviewed in 37) but no systematic epidemiological data exist. Investigation of associations between TB disease and sexually transmitted infections has been strongly dominated by HIV infection, which may obviously be a major confounder. There have been few studies from low HIV prevalence populations. One from China found an association between history of TB and human papilloma virus infection118.
Interestingly, the declining TB mortality rates in The Netherlands and England and Wales in the 20th century showed no surge during the Great Depression105,119, when SES status deteriorated thereby affecting several of these known risk factors, in particular nutritional status. They did however surge during and shortly after the Second World War105,119. In England and Wales this was not paralleled by major deterioration in nutritional status; in The Netherlands famine only started in the winter of 1944–45 while the increase in TB mortality started already from 194237. In both countries during this period major increases were seen in sexually transmitted infections, mainly related to presence of large numbers of Allied and Axis troops37.
Blood transfusion. Transfusion-associated CMV infection occurs in particular following multiple transfusions of whole blood or granulocytes, and can be prevented by removal of white blood cells120–122. Increased incidences of TB have indeed been described in two categories of patients who in the past often received multiple whole blood transfusions: patients who underwent (partial) gastrectomy, mainly for bleeding gastric ulcers123,124, and patients with end-stage renal disease on haemodialysis125. For both these categories alternative explanations for increased TB incidences are possible: low body mass index for gastrectomy123,124, and impaired cellular immunity due to uraemia for haemodialysis126. Nonetheless, several studies among haemodialysis patients have suggested increased rates of CMV (re)infection, either or not associated with transfusion of blood or blood products127–131, as well as increased rates of CMV reactivation127.
Solid organ transplantation. The incidence of symptomatic CMV infection is strongly increased in solid organ transplant patients, mainly due to infection from a CMV IgG positive donor132. Solid organ transplantation also increases the risk of TB disease125,133–135. TB incidence is highest in lung transplant patients and associated with presence of latent TB infection, clinical condition and intensity of the immunosuppressive therapy; the latter has been brought forward as the sole explanation for the increased TB risk136. Interestingly, a study among Korean solid organ transplant patients found that the risk of developing TB was associated with CMV infection within the prior 3 months137.
If indeed CMV (re-)infection or reactivation commonly precipitates progression from latent infection to active TB, this will suggest novel approaches to TB control. Combining a test for Mtb infection with one for ongoing or recent active CMV infection may strongly increase our ability to predict the development of TB disease and allow the targeting of preventive treatment to those most at risk4. Vaccination against CMV might prevent TB in those with TB infection. A wide range of CMV vaccines are currently in clinical development including plasmid-based vaccines, viral vector vaccines, attenuated HCMV strains, and recombinant protein and peptide vaccines138. Recently, a genetically modified CMV vector expressing antigens from Mtb (RhCMV/TB) has shown 41% sterile protection against Mtb in a non-human primate study139. If the human version of this vaccine was able to afford (partial) protection against CMV, it could also significantly impact the TB epidemic. CMV infection may also affect TB treatment response, another potential application therefore could be the provision of CMV antiviral treatment as an adjunct to TB treatment, for example of patients with multidrug resistance.
There is an urgent need for elucidating the role of CMV infection in TB disease progression. Further serological and cellular studies should be done to confirm the association between TB disease and CMV infection. However, in settings with high CMV seroprevalence (also those with highest TB incidence) it will be important to identify recent reinfection for which various diagnostic approaches exist22. Their relative merits are beyond the scope of this article, but some may potentially signal reactivation due to Mtb replication, i.e. consequence rather than cause. Therefore, ultimately longitudinal studies are needed in which the incidence of TB disease among those with latent TB infection is measured over time comparing those with CMV (re-)infection or reactivation to those without. These studies should be supplemented with immunological studies to define the mechanisms through which CMV precipitates progression to active TB disease. Finally, it will be important to study the role of CMV reactivation during TB disease and its effect on the response to TB treatment.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Partly
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: TB Immunologist
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 30 Apr 18 |
read | |
|
Version 1 06 Mar 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)