Keywords
Enterococcus faecalis, Lime (Citrus aurantiifolia) extract, acid tolerance, pH adaptation
Enterococcus faecalis, Lime (Citrus aurantiifolia) extract, acid tolerance, pH adaptation
Enterococcus faecalis is a significant agent in the pathogenesis of root canal infections, especially in post-endodontic treatment, with a prevalence of 24–77% in these infections1. E. faecalis is very difficult to eliminate because the pathogen can survive in poor nutrient conditions. It can adapt to acidic conditions, including living in the dentin tubule of a closed root canal with a smear layer. It can also express the dominant biofilm protein to maintain its attachment to host cells2.
E. faecalis has been shown tolerate to acidic environments as well as to adapt to pH changes, which are the essential virulence factors in maintaining antibacterial balance3. Fisher reported that E. faecalis could survive in environments with high NaCl concentrations at extreme temperatures of 5–65°C with a pH of 4.5–10.04. Stuart et al.1 reported that E. faecalis are less sensitive, with a pH of 5.0 at 25°C after it has been incubated for 10 h. The author also found that it has an excellent growth capability at pH 8.5 and low adhesion at pH 7.1 in a medium coated with bovine serum albumin (BSA).
E. faecalis is resistant to medication materials such as calcium hydroxide5 and chlorhexidine (CHX)6. The long-term use of both medication materials can lead to parachloroaniline (PAC), causing blockage of the dentinal tubules and eventually becoming toxic7. Fosfomycin may also interfere with acid tolerance systems and pH changes of E. faecalis in tooth root canals by inhibiting phosphoenolpyruvate synthetase8.
Indonesia, especially in Aceh has a tropical climate with a variety of plants that can be utilized in medical treatment, including lime extract (Citrus aurantiifolia). It contains phenols, flavonoids, hydrogen peroxide, tannins, alkaloids, and saponins that have antibacterial, antioxidant, antifungal, analgesic, and anti-inflammatory properties9. Nwankwo10 reported that lime extract helped to prevent Klebsiella pneumonia, Salmonella, and Escherichia coli. Here, the acid in lime extract influenced the bacterial development and cell metabolism. The present study evaluates the acid tolerance response and pH adaptation of E. faecalis when it interacts with lime extract.
The lime extract and E. faecalis (ATCC-29212) were used in this study. The extractions were prepared at the Laboratory of Microbiology at the Faculty of Veterinary, University of Syiah Kuala, Darussalam, Banda Aceh, Indonesia. Both materials were made in vitro to analyze the pH adaptation, acid tolerance response, and interaction activity between E. faecalis and lime extracts.
Lime peel was separated from the flesh then dried using dehydrator until the water content reduced to 10%. Dried lime peel was grinded into powders. The powder was put into glass container and masserated with ethanol 70% for two days and then strained using a gauze. Filtrate was evaporated using a rotary evaporator at 80°C to obtain the pure lime extracts.
E. faecalis ATCC 29212 taken from glycerol stock was cultured on a Mueller-Hinton Agar (MHA) medium at a temperature of 80°C (Thermo Fisher Scientific Inc., Paisley, UK). The culture was incubated in anaerobic conditions at 37°C for 48 hours using Anaerogen TM GasPack (Oxoid, Basingstoke UK), and incubator (Memmert, Germany). A colony of E. faecalis bacteria was subsequently re-cultured in 5 ml of Mueller-Hinton Broth (MHB) medium (Thermo Fisher Scientific Inc, Paisley, UK) in anaerobic conditions at a temperature of 37°C for 48 hours. Afterward, the E. faecalis grown on the liquid medium was synchronized further with McFarland 0.5 (1 x 108 CFU/ml) (TM50, Dalynn Biological Inc., Calgary, Canada). The Accurate of the density of McFarland standart can be checked using a spectrophotumeter with an absorbance reading of 0.08 to 0.1 at 625 nm11.
A total of 50 ml of lime extracts in several different concentrations (100%, 75%, 50%, 25%, 12.5%, and 6.25%) was placed into different beaker glasses. Then, 5 ml of E. faecalis in MHB (1:10) were added to each of the beakers. The initial pH of the mixture was measured (0 hours) before incubation. Next, each beaker was incubated at 37°C for 6 hours, 12, hours, 24 hours, 48 hours, and 72 hours in an anaerobic atmosphere using Anaerogen TM GasPack (Oxoid, Basing stoke UK), at each of these times, the beakers' pH was measured using a pH meter (Thermo Fisher Scientific Inc, Paisley, UK). Various changes in pH from 0 hours to the specified time can be used as an indicator of whether E. faecalis has a tolerance response to the acidic environment and can adapt to changing pH12.
The cultures of the pH measurements were used to measure the acid tolerance response of E. faecalis to lime extract utilizing the principle of spectrophotometry13. The analysis was performed based on the incubation time that had been determined following the measurement of pH shaken at 500 rpm. Here, 96 wells of the triple microplate series were coated with 50 μl of MHB (Thermo Fisher Scientific Inc., Paisley, UK) for 15 minutes and then were vacuumed. After that, 100 μl of the test materials (E. faecalis + lime extract) derived from the incubation processes at 6 h, 12 h, 24 h, 48 h, and 72 hours were added to each well. Each well was incubated for 15 minutes to analyze the adaptation of the test materials. Then bio-tolerant activity was measured using the Elisa Reader (Bio-Rad Laboratories, Hercules, CA) at a wavelength of 595 nm.
Adhesion assay was analyzed based on the principles of Gram-staining14. The standard protein concentration of the E. faecalis and the active component concentration of the lime extract were measured via the Bradford method (Bio-Rad, Hercules, California, U.S.A.) using Bovine serum albumin (Merck, Darmstadt, Germany) as the standard protein. Spectrophotometry detected the interaction of E. faecalis with lime extract at a wavelength of 595 nm13,15.
The principle of incubation time-based interaction activity on the microplate 96 wells series used in this research based on Gamble's working principle16; it was modified using violet crystalline and safranin staining. First, 96 wells of the triple microplate series were coated with 50 μl of MHB (Thermo Fisher Scientific Inc., Paisley, UK), settled for 15 minutes, and then aspirated. Second, 50 μL of E. faecalis was added and then incubated for 15 minutes at room temperature. Third, 100 mL of the lime extract was added and incubated for 6 hours, 12 hours, 24 hours, 48 hours, and 72 hours (as adapted from research conducted by Bachtiar)17. All of the residues of the test materials (E. faecalis + lime extract) in the microplate wells were aspirated and then settled for 10 minutes at room temperature. Then, 50 μL of 2% violet crystalline were added to each well for 5 minutes; the wells were washed with phosphate buffer saline (PBS) two times (Merck, Darmstadt, Germany).
A total of 100 μL of Lugol solution was added for 1 minute and then washed with PBS. The rest of the cell metabolism that was not bacterial cells was dissolved in 96% alcohol for 20 seconds until the dye completely removed. 50 μL of safranin solution was added for 2 minutes and then washed again with PBS18. The interaction activity between the lime extract and the E. faecalis bacteria in the microplate wells was assessed via an Elisa reader using a spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA) at a wavelength of 595 nm13.
The anti-adhesion mass of lime extract against E. faecalis that formed on each base of the microplate wells was prepared by adding 100 μl of glycerol for 24 hours to maintain moisture. Visualization was produced by adding 10 μl of immersive oil to each microplate well to be observed under a light microscope at x1000 magnification (Olympus CX21 FS 1, Japan) supported by Optilab Viewer software V 2.2 (Miconus Transdata Nusantara, Jakarta, Indonesia) adapted from a study conducted by Gani19.
E. faecalis acid tolerance and adhesion to lime extract were calculated to determine average values and standard deviations for each concentration. Two-ways analysis of variance (ANOVA) was performed with significance set at p < 0.05. The analysis was performed using SPSS ver. 20.0 software.
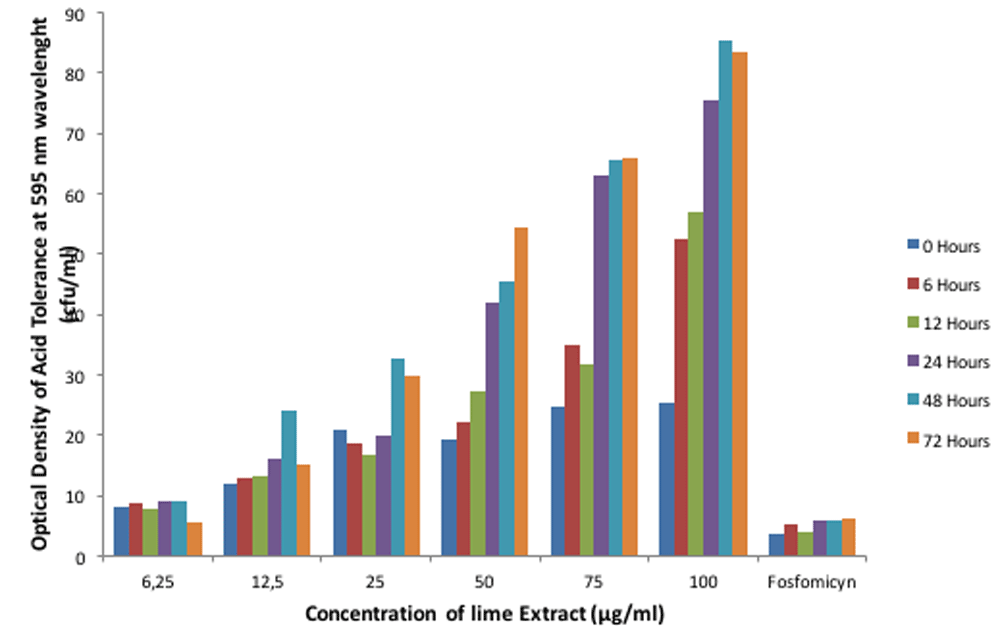
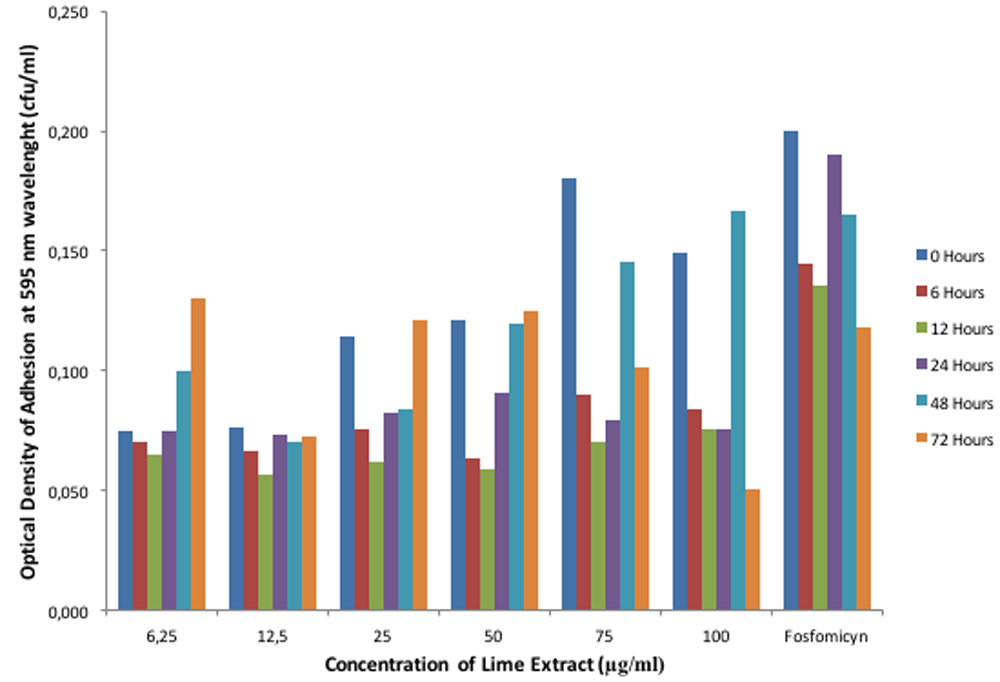
The experiment was conducted in three replicates. The ANOVA test showed that lime extracts and exposed time gave the significant effect on the pH, optical density of acid tolerance respond of E. faecalis in lime extract and optical density of adhesion of interaction activity between E. faecalis and lime extract on biofilm (p<0.05). In addition, the interaction between lime extract and exposed time also gave the significant effect on the pH, optical density of acid tolerance respond of E. faecalis in lime extract and optical density of adhesion of interaction activity between E. faecalis and lime extract on biofilm (p<0.05). The results showed that E. faecalis possessed the ability to adapt to acidic (pH <7) and alkaline circumstances (pH 7>) (Figure 1). Also, E. faecalis is tolerant in the acid of lime extract with different intensities at each concentration (Figure 2). Figure 3 shows that the interaction activity of E. faecalis in lime extract influenced by time and concentration were significant (p<0.01) with strong correlation (0.98). The results showed that the interaction activities of E. faecalis in Lime extract will decrease from 6, 12, 24, and 72 hours.
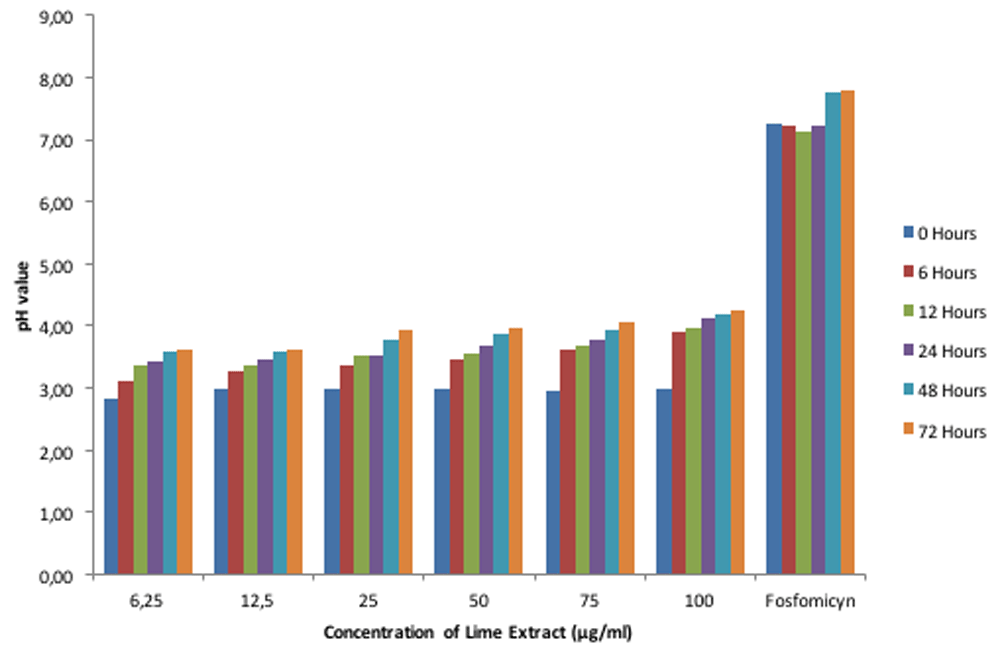
E. faecalis did not express an ability to adapt to the acidic pH of lime extract after interacting with fosfomycin (as a positive control) (Figure 1), although it still expressed acid tolerance response (Figure 2). Its interaction activity was robust (Figure 3). The mass profile also indicated interaction activity between lime extract and E. faecalis. Antibiotics are capable of forming a covalent bond to activate the cysteine residue of a bacterial cell, triggering UDP-N-acetylglucosamine to form hydrogen bonds. It inhibits the synthesis of peptidoglycan as an antibacterial defense.
According to Sitanggang et al.20, the lime extract has a highly acidic pH ranges (1.7–3.1). The acidity is generated by citric acid and amino acids, while the essential oils contribute to maintaining its acidic pH21. Citric acid is reported to play a crucial role as a natural material to maintain pH balance and possesses antibacterial activity22. Figure 1 shows an acidic pH of 2.9 on the extracted bar (without E. faecalis). After E. faecalis was added at various concentrations, there was a significant change (increase) in the acidic pH of lime extract (as a negative control) (p < 0.05). The increased acidic pH of lime extract from 6.25% (µg/ml) to 100% (µg/ml) (see Figure 1) indicates that E. faecalis can adapt to environments with an acidic pH (2.9–4.2) at a temperature of 37°C. Morandi reported that E. faecalis could adapt to situations with a low pH and temperature, although they become less sensitive; however, when adjusted to a pH of 5.0 at 25°C, they display increased sensitivity within 10 hours23,24.
E. faecalis had an acid tolerance response to lime extract that significantly increased as the concentration of the lime extract increased (Figure 2) (p < 0.05). The increased acid tolerance response correlates with the characteristics of Enterococcal strains producing lipoteichoic acids that contribute to biofilm formation and have resistance to antibacterial agents, including tolerance to acidic environmental change25. Molecularly, the acid tolerance response of E. faecalis is influenced by the EfCitH gene, which encodes the citrate transporter protein on the surface of the cell membrane that acts to maintain the balance of the effects of citric acid generated from the environment26. Sarantinopoulos found that enterococcal strains have metabolic potential against the citrate metabolism; this supports their acid tolerance response to environmental influences such as aroma and fermentation products27. In this research, Fosfomycin with a pH of 7.2 (Figure 1) could still slightly tolerate the acidic effects. The acid tolerance response is related to the ability of E. faecalis to grow in environments with an alkaline pH (9.5–12) within 48–72 hours12.
In general, the interaction activity between E. faecalis and lime extract at all concentrations was lower than the interaction activity between E. faecalis and fosfomycin. Due to its size, the lime extract was considered to be stable based on its treatment concentration (Figure 3). Based on the incubation time, the interaction activity between E. faecalis and lime extract at all concentrations within 6–12 hours at the temperature of 37°C was also relatively stable (Figure 3). Varoni et al.28 reported that anti-adhesion activity between plant polyphenol-rich extract and Streptococcus mutans bacteria was at its maximum within 24 hours, while within 6, 7, and 8 hours, the activity was stable but not yet maximal.
Figure 3 shows there was a significant increase in the average concentration bar (p > 0.05), with a relatively high error bar (standard deviation value) within 24–72 hours. It indicates that from 24–72 hours, E. faecalis begins to adapt and tolerate the temperature and pH of the environment. The mechanism utilized by bacteria to survive heat and low-pH of the environment operate in many different ways. The most successful means of surviving low-pH stress is the complete avoidance of extremely acidic environments. However, none more critical than the sensing of mild acidification to prevent the potentially lethal consequences of the inappropriate production of potentially antigenic proteins. Bacteria that are forewarned by mild acidification can prepare through the induction of a wide range of protective measures. It can alter the composition of the cell membrane, extrude protons, protect macromolecules, alter metabolic pathways, and generate alkaline29.
The interaction activity between lime extract and E. faecalis can be assumed to be the antibacterial activity, because there was a decrease in the interaction activity between E. faecalis and the biological components of lime extract within 6–24 hours and again within 48–72 hours. The interaction activity is related to the activity of the active ingredients contained in the lime extract, such as flavonoids (polyethoxylated flavones and flavanones), coumarin, and terpenoids, all of which act as antibacterials30,31.
E. faecalis can adapt to environments with a pH of 2.9–4.3 generated by lime extracts. In addition E. faecalis also expressed a tolerance response to the acidic environment. The interaction activity between E. faecalis and lime extract become stable within 6–12 hours at a temperature of 37°C. Therefore, the lime extract can be used to inhibit the E. faecalis growth.
Dataset 1: pH adaptation of E. Faecalis in lime extract based on replications 10.5256/f1000research.13990.d19664332.
Dataset 2: Optical density (OD) of acid tolerance respond of E. Faecalis in lime extract based on replications 10.5256/f1000research.13990.d19664433.
Dataset 3: The OD value of the interaction activity of E. Faecalis in lime extract based on replications 10.5256/f1000research.13990.d19664634.
We would like to thank the Laboratory of Microbiology at the Faculty of Veterinary, Syiah Kuala University, Darussalam, Banda Aceh, Indonesia for preparing the E. faecalis ATCC 29212 and lime extract as the test materials used in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 11 Apr 18 |
read | |
|
Version 1 07 Mar 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)