Keywords
lithium, treatment response, gene expression, machine learning, microarray, transcriptome, precision medicine, pharmacogenomics
This article is included in the Artificial Intelligence and Machine Learning gateway.
lithium, treatment response, gene expression, machine learning, microarray, transcriptome, precision medicine, pharmacogenomics
See the authors' detailed response to the review by Sunil V. Kalmady
See the authors' detailed response to the review by Ming-Fen Ho
Lithium is the most well-established mood-stabilizer in the practice of psychiatry (Jermain et al., 1991; Landersdorfer et al., 2017). A recent propensity-score adjusted and matched longitudinal cohort-study evaluating the effectiveness of the newer mood stabilizers: olanzapine (n=1477), quetiapine (n=1376), and valproate (n=1670), in comparison to lithium (n=2148), found that patients treated with lithium experienced reduced rates of both unintentional injury and self-harm (Hayes et al., 2016). However, due to lithium’s narrow window, 0.5–1.2 mEq/mL, of maximal effectiveness and safety (i.e. therapeutic index), Therapeutic Drug Monitoring is the standard-of-care to ensure patient safety in medical practice (Hiemke et al., 2011). Further, divergent clinical response rates have been reported among male and female patients diagnosed with bipolar disorder and treated with lithium (Viguera et al., 2000).
In a 1986, Zetin and colleagues published the results of a study that evaluated four methods for predicting lithium daily dosages, and the final equation resulted in a 147.8mg/day increased dosage-adjustment for male patients (Zetin et al., 1986). Similarly, a later study by Lobeck and colleagues corroborated the 147.8 mg/day male increase dose requirement for the lithium maintenance dose in bipolar patients (Lobeck et al., 1987). However, neither do the current dosing guidelines recommend a gender-based dose adjustment via clinical pharmacometrics, to avoid toxicity, nor are gender-specific gene expression screening panels available to predict lithium efficacy currently available and implemented.
A recent large-scale meta-analysis of human body-tissue gene expression reported that the body organ with the most abundant gender-biased gene expression is the anterior cingulate cortex within the frontal cortex of the brain (Mayne et al., 2016). Thus, these findings suggest that therapeutic drug response may be influenced not only via drug absorption, distribution, metabolism, and elimination, but also within the underlying gene signatures across the human transcriptome and mechanisms of gene-gene interactions that regulate physiology. Beech and colleagues conducted a study to identify gene expression differences from the peripheral blood in patients classified as lithium responders and non-responders (Beech et al., 2014). However, the study reported that no significant gender-biased gene expression differences were found (p-value=0.941) in patients who were randomized to optimal therapy (control), defined as one FDA-approved mood stabilizer, versus patients treated with lithium plus optimal therapy (Beech et al., 2014). Despite these initially reported findings, a recent study by Labonté and colleagues, which used RNA-Seq to evaluate the transcriptome in patients diagnosed with major depressive disorder (MDD), concluded that gender dimorphism exists at the transcriptome-level in MDD patients and that gender-specific treatments should be investigated (Labonté et al., 2017).
Therefore, there is an urgent clinical need to improve behavioral healthcare by understanding gene expression variability that may lead to personalizing medicine in patients with mania. These findings may improve prediction of clinical drug response of lithium prior to initiating pharmacotherapy in patients with bipolar or schizoaffective disorders, who cannot risk drug inefficacy for obvious safety reasons. Therefore, the overall aim for our study is to define gender-specific transcriptional-level regulators of lithium treatment response that may influence treatment of bipolar or schizoaffective disorders. We will test the hypothesis that biologically plausible gene expression differences exist, prior to lithium treatment, in patients diagnosed with bipolar disorder in the following three patient subgroups: (1) male and female patients who were later clinically classified as lithium treatment responders; (2) male-responders versus male-non-responders; (3) female-responders versus female-non-responders.
DNA microarray data analyzed in this study are originally referenced from the Lithium Treatment-Moderate dose Use Study placed in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) via accession number GSE45484 with the Illumina HumanHT12 V4.0 expression Beadchip GPL10558 platform file to associate gene names and descriptions. The original multisite clinical study recruited patients from Case Western Reserve University, Massachusetts General Hospital, Stanford University, Yale University, and the Universities of: Pittsburgh, Texas Health Science Center at San Antonio, and Pennsylvania (Beech et al., 2014). From the original 120 peripheral blood samples used to generate probe and gene expression profiles, from patients diagnosed with bipolar disorder, the clinical phenotype of being either a treatment- responder or non-responder was assessed using the Clinical Global Impression Scale for Bipolar Disorder-Severity (CGI-BP-S) (Spearing et al., 1997).
To assess for gender-specific differential gene signatures, in our first analysis we grouped patients based on gender alone and not on any other variables (i.e. optimal treatment versus lithium, or responder versus non-responder status). Then, we rationalized that from the results of the gender-specific transcriptome signatures from our first analysis, we will set the top two-hundred and fifty genes as controls in an effort to identify pharmacologic treatment-response transcriptome biomarkers that are not directly linked to the X or Y chromosome. Therefore, we excluded the top two-hundred and fifty genes from all results that were reported in subsequent analyses to identify genes with lithium-specific transcriptional differences between genders associated with response treatment. In our second analysis, we only selected patients who were classified as lithium treatment-responders, at baseline, and the results from the gene expression differences are reported excluding the sex-specific control genes identified in the first experiment. In our third and fourth analyses, we compared: male-responders vs. male non-responders, and female-responders vs. female non-responders, respectively.
Differential gene expression analysis of the microarray data was conducted using the Empirical Bayes method implemented within the limma package (version 3.34.5) and utilizes the Biobase package (version 2.38.0) which both run within the R for Statistical Programming environment (version 3.4.3; R Foundation for Statistical Computing, Vienna, Austria) (Ritchie et al., 2015; Team, 2013). Due to multiple testing of the peripheral blood transcriptome, the False-Discovery Rate was adjusted using the Benjamini-Hochberg method. The Decision Tree and Random Forests machine learning algorithms were used to assess gender using transcriptional signatures and for predictive modeling using the discovered microarray genes to select for the gender-specific lithium responders. A p-value of less 0.05 was considered to be statistically significant and a differential gene expression threshold of 0.5 was used and reported during the machine learning process. Further methods detailing the Random Forests decision processes for male- and female-responders are located in Supplementary File 1.
Table 1 provides the patient age and sample sizes used during subgroup analyses. In our first analysis, which aimed to group patients based on gender alone and not based on clinical variables detailed in the original study, data-driven gene analytics identified four female-labeled patient samples with gene expression levels similar to that found in male patients for the following Y-chromosome genes: RPS4Y1, EIF1AY, KDM5D, RPS4Y2; and the XIST gene located on the X-chromosome. Therefore, all subsequent hypothesis-testing were analyzed with the updated male-gender classification for the following NCBI GEO patient samples: GSM1105526 (baseline lithium-non-responder), GSM1105528 (1-month lithium-non-responder), GSM1105546 (baseline lithium-non-responder), and GSM1105548 (1-month lithium-non-responder). Figure 1 illustrates the gene expression findings resulting in re-assignment for the aforementioned patient samples from females to males using a decision-tree approach that evaluated if the RPS4Y1 gene had an expression level of greater than or equal to 9.6 resulting in: yes=male (31%) and no=female (69%). After proceeding with the machine learning analysis of both the ‘training’ and ‘validation’ datasets, the final ‘test’ dataset resulted in the following diagnostic test evaluation parameters: Sensitivity=100% (95% C.I. 66.37%-100.00%), Specificity=100% (95% C.I. 78.20%-100.00%), and an area under the receiver operator characteristic (ROC) curve of 1. Figure 2 illustrates the variable importance plots used in the machine learning process for classifying patients as being a male-lithium-responder or female-lithium-responder relative to the full patient population. The results show, in descending order of predictive power, the genes selective for male lithium-responders versus the full patient population being RBPMS2, CDH23, and SIDT2. Similarly, in descending order of predictive power, for female lithium-responders versus the entire patient population, the FHL3, ABRACL, RPL10A, and RPS23 genes are most selective.
| Lithium treated patient population | |||
|---|---|---|---|
| Baseline | Mean age | S.D. | Sample size (n) |
| Male-responder | 36 | 8.1 | 3 |
| Female-responder | 31 | 11.8 | 6 |
| Male-non-responder | 40 | 10 | 7 |
| Female-non-responder | 44 | 9.2 | 12 |
| *General mood stabilizers patient population | |||
| Baseline | Mean age | S.D. | Sample size (n) |
| Male-responder | 51 | -- | 1 |
| Female-responder | 49 | 10.5 | 3 |
| Male-non-responder | 43 | 12.5 | 9 |
| Female-non-responder | 37 | 14.5 | 19 |
| Total patient population | |||
| Gender | Mean age | S.D. | Sample size (n) |
| Male | 41 | 10.8 | 20 |
| Female | 39 | 13.1 | 40 |
| Study population | 40 | 12.3 | 60 |
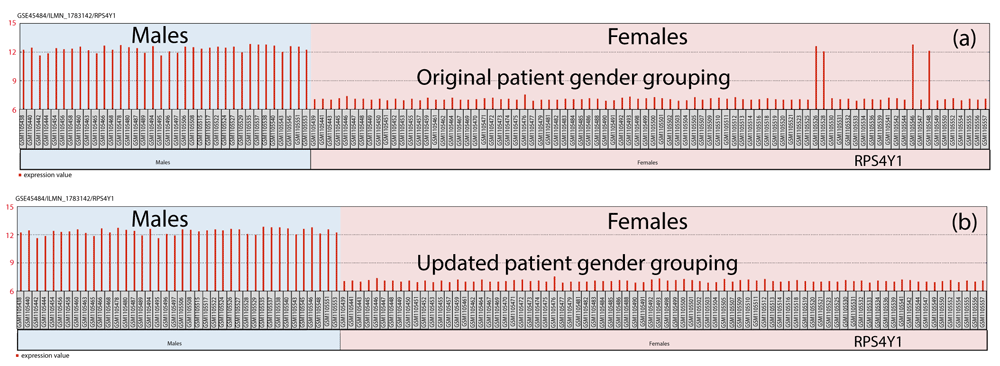
Males (n=20; with 40 pre- and post-treatment samples) and Females (n=40; with 80 pre- and post-treatment samples).
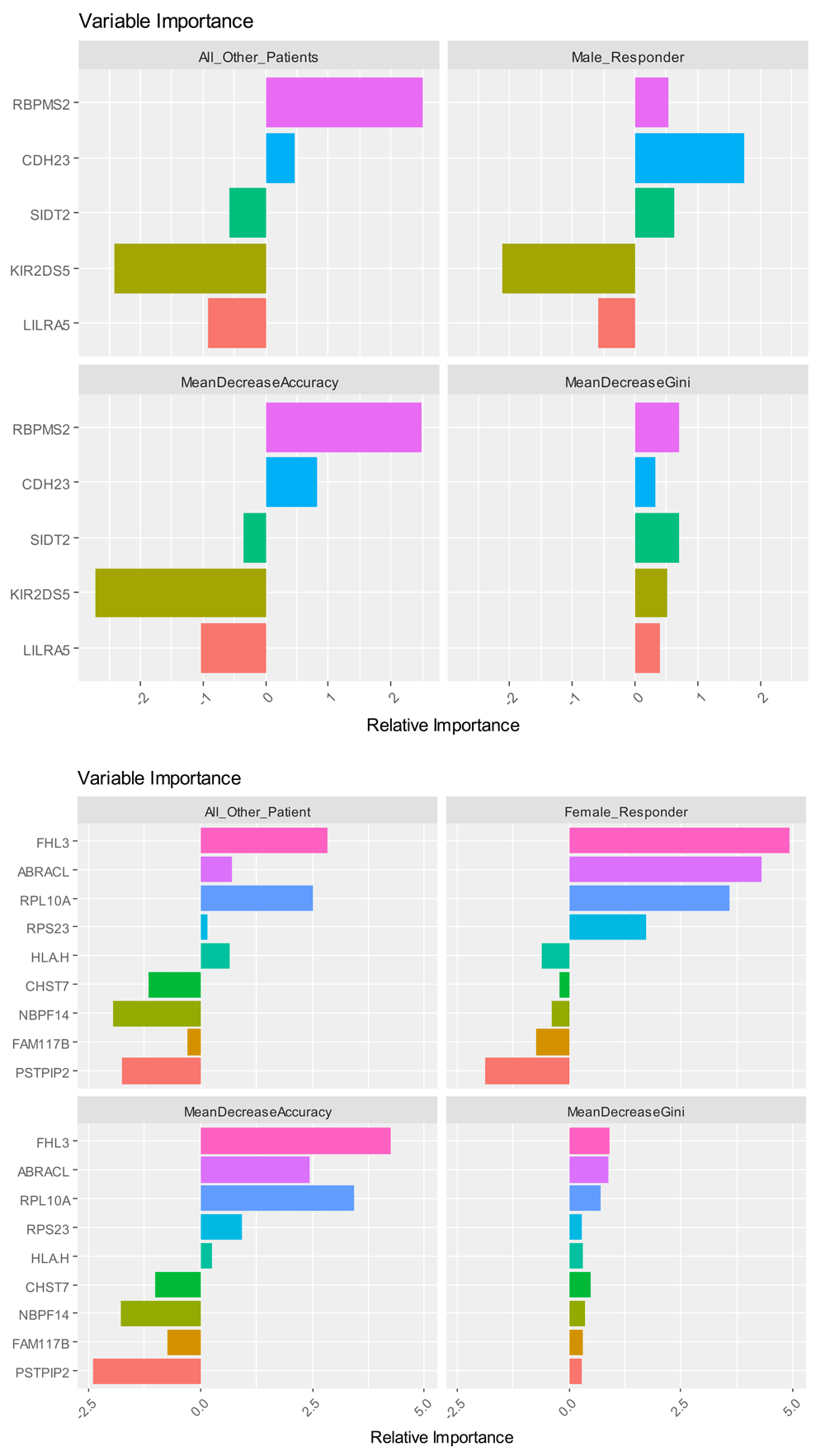
Table 2 provides the results for the gender-specific differentially expressed genes from the entire study population using a fold-change (FC) threshold of 0.5. A total of five genes met the a priori FC requirements and were found to be RPS4Y1, EIF1AY, KDM5D, RPS4Y2, and EIF1AY. These five down-regulated male-biased genes were all found on the Y-chromosome. Contrastingly, a total of 10 upregulated female-biased genes were found to be: XIST, S100P, IFIT3, TNFAIP6, IFITM3, IFIT2, CHURC1, ANXA3, ADM, and PROK2. The RPS4Y1 gene in males (FC= -4.9807, p=7.36E-47) and the XIST gene (FC=1.7615, p=2.98E-36), found on the X-chromosome, in females resulted in the greatest expression changes between genders. The male-favored genes resulted in a larger expression change than compared to the females.
Table 3 provides the results for the differentially expressed genes that were found between male and female responders prior to initiation of lithium and optimal therapy, meeting the FC criteria of at least 0.5. In male lithium responders, we found 5 differentially expressed while the RNA binding protein with multiple splicing 2 (RBPMS2) gene ranked with the greatest FC of -1.351 (unadjusted p=0.00111). Whereas, 9 genes were associated with female lithium responders, with greatest expression change being the major histocompatibility complex class-1-H (HLA-H) at 1.602 (unadjusted p-value=0.00099). The neuroblastoma breakpoint family member-14 (NBPF14) gene met the Benjamani-Hochberg adjusted p-value criteria and resulted with an expression change of 0.586 (adjusted p=0.0462). Figure 3 illustrates the heat-map and dendrogram overview of the two-way unsupervised hierarchical cluster analysis of the reported differentially expressed genes among male and female responders to lithium therapy at baseline that correspond to values reported in Table 3.
| Genes associated with male lithium responders | |||||
|---|---|---|---|---|---|
| Gene | Adjusted P-value | P-value | Log fold change | Gene description | Highest gene tissue expression |
| RBPMS2 | 1 | 0.00111 | -1.351 | RNA Binding Protein with Multiple Splicing 2 | Heart, Urinary Bladder |
| SIDT2 | 1 | 0.00932 | -0.82 | SID1 Transmembrane Family Member 2 | Stomach, Prostate |
| CDH23 | 1 | 0.00388 | -0.674 | Cadherin-Related 23 | Ovary, Fat |
| LILRA5 | 1 | 0.00359 | -0.592 | Leukocyte Immunoglobulin Like Receptor A5 | Appendix, Bone Marrow |
| KIR2DS5 | 1 | 0.00431 | -0.506 | Killer Cell Immunoglobulin Like Receptor, Two Ig Domains and Short Cytoplasmic Tail 5 | -- |
| Genes associated with female lithium responders | |||||
| Gene | Adjusted P-value | P-value | Log fold change | Gene description | Highest gene tissue expression |
| HLA-H | 1 | 0.000996 | 1.602 | Major Histocompatibility Complex, Class I, H (pseudogene) | Lymph Node, Bone Marrow |
| RPS23 | 1 | 0.00308 | 1.471 | Ribosomal Protein S23 | Ovary, Bone Marrow |
| FHL3 | 1 | 0.000751 | 0.893 | Four and a Half LIM Domains 3 | Esophagus, Endometrium |
| RPL10A | 1 | 0.00299 | 0.628 | Ribosomal Protein L10a | Ovary, Bone Marrow |
| **NBPF14 | **0.0462 | 0.00000782 | 0.586 | Neuroblastoma Breakpoint Family Member 14 | Skin, Ovary |
| PSTPIP2 | 1 | 0.000473 | 0.569 | Proline-Serine-Threonine Phosphatase Interacting Protein 2 | Bone Marrow, Spleen |
| FAM117B | 1 | 0.00949 | 0.556 | Family with Sequence Similarity 117 Member B | Testis, Adrenal |
| CHST7 | 1 | 0.00812 | 0.529 | Carbohydrate Sulfotransferase 7 | Spleen, Fat |
| ABRACL | 1 | 0.00396 | 0.505 | ABRA C-Terminal Like | Colon, Lymph Node |
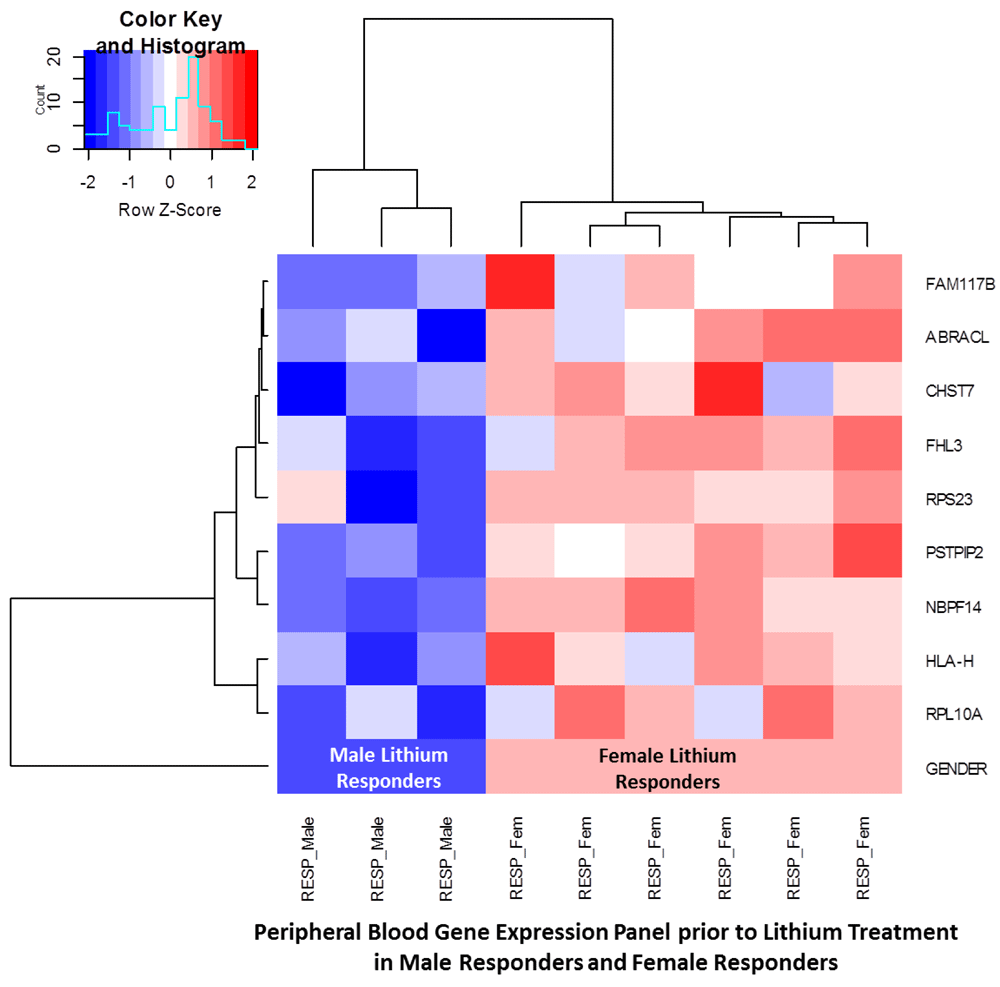
Using the baseline blood sample microarray data, the predictive modeling results for identifying lithium-responders from the complete study population of male and female controls and treatment samples, resulted in a validation/test sample cohort for males of: Sensitivity=95.83% (95% C.I. 78.88%-99.89%), Specificity=not calculated due sample size of test dataset, and an ROC curve AUC = 0.92 using the RBPMS2 and LILRA5 genes. Likewise, in the test dataset for females: Sensitivity=91.67% (95% C.I. 61.52%-99.79%), Specificity= not calculated due sample size of test dataset, and an ROC curve AUC = 1 with the ABRACL and NBPF14 genes. Therefore, we developed a 2-gene predictive model for men and likewise for women predicting lithium response in bipolar patients from a general population of bipolar patients using transcriptional signatures at baseline.
Table 4 provides the list of 10 differentially expressed genes found in male lithium responders (5-genes) and male lithium-non-responders (5-genes). The RNA binding protein with multiple splicing 2 (RBPMS2) gene (FC= -1.326, unadjusted p=0.001358) in male lithium responders and the Ribosomal protein S23 (RPS23) gene (FC=1.521, unadjusted p=0.013306) were found to result in the largest expression change differences between subgroups. However, in female responders and female non-responders, the Family with Sequence Similarity 117 Member B (FAM117B) gene (FC=0.5257, unadjusted p=0.0048554) and the Golgin B1 (GOLGB1) gene (FC= -0.6536, unadjusted p=0.0003716) were differentially expressed, respectively and shown in Table 5.
The purpose of this investigation was to define gender-specific transcriptome-level regulators of lithium treatment response prior to the initiation of lithium treatment. We first established the gender-relevant transcriptional control genes across all study-participant blood samples and specifically to male- and female-responders using a differential gene expression threshold of 0.5. We found this to be adequate and corroborated with similar studies that used a similar threshold for establishing gene transcription signatures (Jansen et al., 2014; Mayne et al., 2016). However, when comparing the male-responders to male non-responders, as well as, the female responders to female non-responders, we set an inclusion fold-change threshold to 0.3. This approach is not unusual, since it is already established that both large and subtle expression changes produce to significant biological and physiological processes (Wurmbach et al., 2002). Our analysis is both hypothesis-generating, and establishes a computational methodology that provides insight to the importance of subgroup analysis in genomic medicine, irrespective of patient sample-sizes. The end-goal of such analyses serves as a testing methodology for establishing gene screening panels to improve personalized medicine in vulnerable and high-risk patient populations. In these patient populations, it is often not feasible to wait for weeks to determine whether a prescribed medication will work and in some cases manic patients are neither able to fully comprehend and be objectively assessed using the CGI-BP-S (Spearing et al., 1997).
When reviewing the heat-map and dendrogram hierarchical cluster analysis patterns, specifically the numerous non-responders clinically-labeled and illustrated in Figure 4, they suggest that the underlying etiology resulting in clinical symptoms (e.g. mania) that led to the diagnosis of bipolar disorder may need re-classification. Further, the subsequent treatments may need to be tailored in data-driven computational psychiatry approaches. In Figure 4, for the females, the samples in the center cluster illustrates that a group of patients are clear non-responders while the patients clustered in the far-right are partial-responders, from a molecular perspective. The natural questions that arise are: (1) How to best convert the non- and partial-responders to treatment-responders? (2) Is a behavioral intervention, in this select group of patients, for whom lithium is not effective, the best answer because the symptoms maybe of a different etiology? If indeed the symptoms are of a different etiology (e.g. inflammatory), from the lithium treatment-responders, then other diagnostic (e.g. electrophysiological neuroimaging) tools may be warranted and corresponding most efficacious treatments sought.
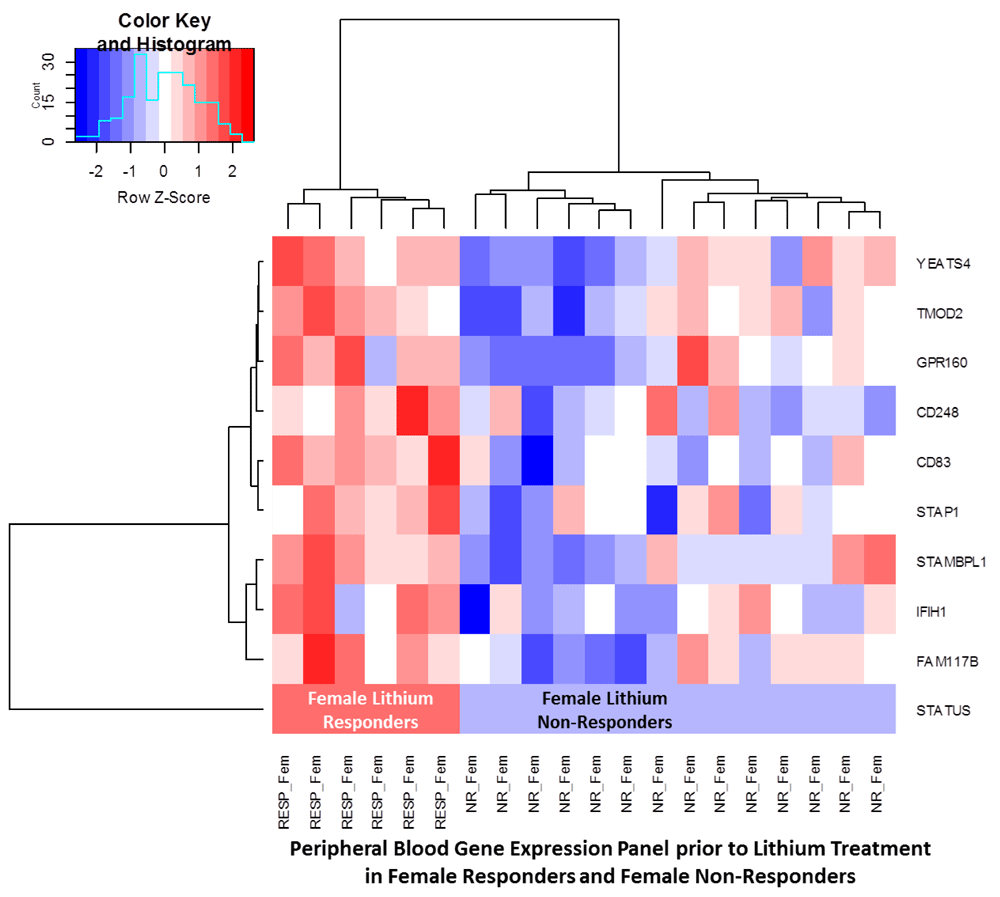
When differentiating between male and female patients, we found that the Ribosomal Protein S4, Y-linked 1 (RPS4Y1, adjusted p-value=7.36E-47) male-linked gene and the X Inactive Specific Transcript (XIST, adjusted p-value=2.98E-36) female-linked gene were the most differentially expressed among genders, which is consistent with previously published studies (Guillén et al., 2014; Jansen et al., 2014; Mayne et al., 2016). The genes that are specific to male lithium responders, relative to female lithium responders, are RBPMS2, SIDT2, CDH23, LILRA5, and KIR2DS5. Using the same methodology, genes identifying female lithium responders, relative to male lithium responders, are HLA-H, RPS23, FHL3, RPL10A, NBPF14, PSTPIP2, FAM117B, CHST7, and ABRACL. The Neuroblastoma Breakpoint Family Member 14 (NBPF14, adjusted p-value=0.0462, Fold-change=0.586) achieved the Benjamani-Hochberg adjusted p-value of 0.0462, and has been reported to be associated with cortical neurogenesis (Suzuki et al., 2017).
Computational psychiatry, as advocated by the National Institute of Mental Health’s Research Domain Criteria (RDoC), may need data to drive the classification, diagnosis, and treatment response status, especially in patients with developmental delay, language difficulty, and condition of a potentially different etiology than traditionally taught (Clark et al., 2017; Eugene & Masiak, 2016). Ideally, in such cases, alternative FDA-approved mood stabilizers may be initially selected prior to any pharmacological intervention by simply using a blood test. Perhaps, a gene expression screening panel at baseline, prior to the initiation of lithium and/or other FDA-approved mood stabilizer, may be better in high-risk patient populations.
These findings suggest that when implementing genomic medicine, clinical research teams should move beyond the single-gene approach when screening for treatment responders or non-responders. This approach is currently the standard when screening for patient toxicity at standard doses in poor or ultra-rapid metabolizers; however, as more transcriptional factors are discovered that regulate the cytochrome (CYP) P-450 system of genes, multi-gene pharmacokinetic panels are inevitable and may be included in future Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines. Next, medical management of patients with mania and psychosis either with pharmacotherapy and/or behavioral intervention should be tailored to biological gender due to known neuronal circuitry differences in age-matched patients with psychosis (Eugene et al., 2015). Further, as a result of lithium not being hepatically metabolized, but rather transported and renally excreted, as well as, the known myriad drug-drug interactions, patient dose selection may benefit from clinical pharmacometrics modeling by American Board of Clinical Pharmaology certified physicians in applied clinical pharmacology/clinical pharmacology (Perera et al., 2014; Zetin et al., 1986). This approach may be implemented to ensure drug pharmacokinetic safety.
The limitations of our analysis and in most genetic studies are understandably due to multiple-comparison p-value adjustments and patient sample size (Dudoit et al., 2003). The fundamental aims of our research questions were designed to answer biological questions of gender and clinical response to lithium and not meant to be driven exclusively by multiple comparisons adjusted p-values. This approach has led to various successes in genomic medicine, specifically, in genome-wide association studies; however, understandably, the limitations are thoroughly acknowledged. In reference to patient sample sizes, 9 out of the 28 patients who received lithium and optimal therapy were classified as lithium treatment responders. Further, 30% of men and 33% of women, who were treated with lithium, were found to be responders at the respective gender categories (Beech et al., 2014). However, the strengths of our findings are in the gender-gene screening ability for lithium treatment-responders in the general population of 60 patients at baseline, minus the tested responder group. Opportunities exist for prospective clinical trials and application of the methods outlined in this text for other therapeutic agents across several medical specialties.
We explored the Lithium Treatment-Moderate dose Use Study clinical trial gene expression data with the aim of identifying gender-specific transcriptome-level regulators of lithium treatment response. Using machine learning, we successfully developed a pre-treatment gender- and gene-expression-specific predictive model selective for lithium responders with an ROC AUC of 0.92 for men and an ROC AUC of 1 for women. Further, by using well-established Bayesian statistical methods, to identify differentially expressed genes and then machine learning, we discovered 5-genes selective for men and 9-genes that are selective for women that will inform the physicians and clinical staff of whether the patient will respond to lithium prior to being prescribed the drug. With the small number of patient responders from the clinical trial, our results should be confirmed. Lastly, in an overall context, our results suggest that the methodology used in this analysis may be extended to other therapeutic drug classes and provides insight to the gender-based gene transcriptome differences influencing lithium pharmacodynamics.
Data used in this study are available from https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45484
The authors gratefully acknowledge the patients in the original clinical trial, the medical staff, and the NCBI GEO database accession GSE4548.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Psychiatric research, Biostatistics, Machine learning.
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 07 Dec 18 |
read | read | |
|
Version 2 (revision) 29 May 18 |
read | read | |
|
Version 1 18 Apr 18 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)