Keywords
non-HDL-c, LDL-c, cholesterol, ASCVD, risk factor, Coronary Artery Disease
non-HDL-c, LDL-c, cholesterol, ASCVD, risk factor, Coronary Artery Disease
Atherosclerotic cardiovascular disease (ASCVD) is the most common cause of morbidity and mortality in the world, representing 31.5% of deaths, with approximately 17.3 million deaths globally1. Hyperlipidemia plays an important role in the pathogenesis of atherosclerosis by inducing chronic inflammation, arterial plaque formation and remodeling, leading to compromised perfusion. Thankfully, hyperlipidemia remains a modifiable risk factor for ASCVD2,3.
Historically, the therapeutic goal for ASCVD risk reduction was to reduce cholesterol levels associated with low density lipoproteins (LDL-c), as elevated quantities have been associated with a higher incidence of ASCVD4. An important body of evidence, including randomized controlled trials, have demonstrated that statins reduce mortality from ASCVD when used as primary or secondary prevention5–8. Nonetheless, other studies have shown that the risk for future cardiac events remain elevated despite achieving LDL-c goals, suggesting that LDL-c might not be the best estimator of ASCVD in some populations9,10.
LDL-c levels only reflect the amount of cholesterol contained within the low density lipoproteins, but does not quantify its quantity, size or structure. Additionally, there are other lipoproteins that possess atherogenic properties, such as very low density lipoproteins (VLDL-c), chylomicrons, and lipoprotein remnants. All these have Apo-B, and can participate in atherogenesis by accumulation in the intima and eliciting pro-inflammatory responses11. Other disadvantage of using LDL-c is the methodologic limitation of its calculations using Friedewald´s equation, which cannot be used in the setting of hypertriglyceridemia12. Recall that elevated triglycerides (TGs) can independently increase the risk for ASCVD13. Therefore, non-high density lipoprotein (HDL) cholesterol has emerged as an alternative predictor of ASCVD.
Non-HDL cholesterol essentially represents the sum of all lipoproteins that have atherogenic properties (LDL, VLDL, IDL, lipoprotein remnant)11. Studies such as the Emerging Risk Factors Collaboration14 (N=302,430) suggest that aiming to reduce non-HDL disregarding other lipid parameters might be a new and better approach. This is supported by the fact that patients in this study with elevated non-HDL-c had higher risk of cardiac events (HR=1.50; CI 95%=1.39–1.61) than those with elevated TGs (HR=0.99; CI 95%=0.94–1.05) or with elevated LDL-c (HR=1.38; CI 95%=1.09–1.73). Moreover, non-HDL-c has demonstrated to be a useful predictor for the appearance of metabolic syndrome, which can be of great utility in primary care settings15. Lastly, non-HDL-c seems to be a better predictor of metabolic syndrome compared with LDL-c, even in patients with TG <400 mg/dL, and the predictive value was independent from central obesity and insulin resistant states16.
Despite all the advantages of non-HDL-c in order to estimate ASCVD risk, the current practice measurement of non-HDL-c is underused. The objective of this study was to evaluate the predictive value of non-HDL-c for the aggregation of multiple ASCVD risk factors and compare it with LDL-c in general subjects with normal or near normal TGs from Maracaibo municipality in Venezuela.
The Maracaibo City Metabolic Syndrome Prevalence Study (MMSPS) is a descriptive and cross-sectional study carried out by our research group in Maracaibo, Venezuela, with the main goal to determine the prevalence of metabolic syndrome in this population and it´s methodology was described previously17. For the purpose of the present sub-study, individuals with no determination of fasting insulin level were excluded; thus, a total of 2026 individuals older than 18 years old were included for this investigation. The study was approved by the Bioethics Committee of the Endocrine and Metabolic Diseases Research Center – University of Zulia (approval number: BEC-006-0305). This ethical approval included all future studies that used the data from the MMSPS. All participants signed written consent before being questioned and physically examined by a trained team.
All individuals underwent a full history and physical exam by trained personnel. During the initial interview, personal and family history of premature ASCVD, endocrine and metabolic diseases were explored. Age, gender, as well as social and economic stratus using Graffar’s scale modified by Mendez-Castellano18, were recorded. Smoking history was categorized in three different classes: a) current smoker (smoked >100 cigarettes in a lifetime, current smoking, and chronic smoker who stopped for <1 year; b) ex-smoker (smoker who stopped smoking for >1 year); c) non-smoker (never smoked or who smoked <100 cigarettes in a lifetime). Current drinkers were considered to be those having drunk >1 gram a day20.
Physical activity was assessed by the Long Form of the International Physical Activity Questionnaire (IPAQ)21. This instrument quantifies the amount of minutes invested in transportation, work, homework (gardening, cleaning), and leisure time. The participants were divided into quintiles based on total Metabolic Equivalents (METs)/min/week scores considering a sedentary person those with a MET score of 0 and those individuals with some degree of physical activity (≥1 MET) were stratified into five groups: very low (Q1), low (Q2), moderate (Q3), high (Q4) and very high (Q5) for a total of six categories. Leisure time was classified as follows: Q1 or very low PA in men<296.999 METs and women <230.999 METs; b) Q2 or low PA in men 297.000–791.999 METs and women 231.000–445.499 METs; c) Q3 or moderate PA in men 792.000–1532.399 METs and in women 445.500–742.499 METs; d) Q4 high PA in men 1532.400–2879.999 METs and in women 742.500–1798.499 METs; and e) Q5 or very high PA in men ≥2879.000 METs and women ≥1798.500 METs.
Blood pressure was measured by manual methods using a sphygmomanometer and stethoscope to detect 1st and 5th Korotkoff’s sounds for systolic and diastolic blood pressure, respectively. Participants had a 15 minute resting period before BP determination, they were seating with both feet on the ground. Measurements were repeated three times in 15 minute intervals. Joint National Committee 7 (JNC7) was used to classify BP as normal BP <120/80 mmHg, prehypertension in those with systolic blood pressure (SBP) 120–139 mmHg and/or diastolic blood pressure (PAD) between 80–89 mmHg, and hypertension when BP is ≥140/90 mmHg22.
Height was determined using a calibrated stadiometer placed on a flat surface. Weight was determined using a digital scale (Tanita, TBF-310 GS Body Composition Analyzer, Tokyo – Japan), with the patient wearing light clothing and barefoot. Body mass index (BMI) was determined using Quetelec´s equation [weight/height2], and using World Health Organization criteria participants were deemed normal weight (BMI <25 kg/m2), overweight (25.0 – 29.9 kg/m2), and obese (≥30.0 kg/m2)23.
Waist circumference was measured using a standardized metric belt using the metric system in centimeters and millimeters. An anatomic reference was used to measure waist circumference an equidistant point between the lower border of the ribs and the antero-superior iliac spine, according to the National Institutes of Health of the United States24. Central obesity was considered if waist circumference was ≥91 cm in women and ≥98 cm in men, according to the specific cut off values proposed for the population of Maracaibo, Venezuela25.
Antecubital venous sampling was performed after an eight hour period of fasting. Samples were centrifuged and serum was obtained. Levels of glucose, total cholesterol, and TGs were determined using commercial enzymatic and colorimetric ELISA kits (Human Gesellshoft Biochemica and Diagnostica MBH). Glucose levels were interpreted according to the American Diabetes Association 2017 diagnostic criteria as follows: normal glucose <100 md/dL, impaired fasting glucose when fasting glucose is 100–125 mg/dL, and diabetes mellitus when glucose was ≥126 mg/dL26. Before diagnosing diabetes, a confirmatory test was repeated on a different day. Levels of high sensitive C reactive protein (hs-CRP) were determined using immunoturbidimetric analyses (Human Gesellshoft Biochemica and Diagnostica MBH), and the cut off point for an elevated hs-CRP was ≥0.765 mg/L27.
Fasting insulin concentration was determined using a commercial kit based on ELISA (DRG International. Inc. USA. New Jersey), with a detection limit of <1 mU/L. Resistance to insulin was calculate by the software HOMA-Calculator v2.2.2 provided by the Oxford Centre for Diabetes Endocrinology and Metabolism. Cutoff value for HOMA2-IR was 2.0028.
Non-HDL cholesterol levels were calculated with the following formula:
Non-HDL-c = total cholesterol – HDL-c
LDL-c were determined using Friedwald formula29. Cutoff points for non-HDL-c: a) <130 mg/dL; b) 130–159 mg/dL; and c) ≥160 mg/dL. Cutoff points for LDL-c: a) <100 mg/dL; b) 100–129 mg/dL; and c) ≥130 mg/dL30.
The aggregation of multiple risk factors was considered when one individual presented with two or more of the following:
Qualitative variables were shown as absolute and relative frequencies. Associations between these variables were explored using χ2 (Chi square) testing and differences with Z test. Quantitative variables were shown as arithmetic mean ± standard deviation after normality testing was performed using the Geary test. Non-normal distribution variables were logarithmically transformed and analyzed as with parametric testing when normality was achieved. When these variables remained non-normal they were shown as median with interquartile ranges (p25–p75th). U Mann Whitney test and Kruskal-Wallis test were used for comparisons between two groups and three or more groups, respectively.
A multivariate regression model was created to estimate odds ratio and confidence intervals for prediction of composite of multiple risk factors. The first model was adjusted for age, sex, age group, ethnic group, socio-economic status, literacy, employment status, smoking, alcohol consumption, physical activity during leisure time, hypertension, hs-CRP, LDL-c and non-HDL cholesterol.
SPSS v.21 for Windows (IBM Chicago, IL) was used for statistical analyses and data gathering. We considered results statistically significant at p<0.05.
From the 2026 participants, 52.1% (n=1056) were female and 47.9% were male (n=846). The mean age was 40.79±15.76 years. Other general features are presented in Table 1. Median non-HDL-c was 143 mg/dL (114–174) mg/dL, with 144 (115–174) mg/dL among females and 143 (114–174) mg/dL in males; p=0.740.
Table 2 shows the epidemiology of non-HDL-c according to social and demographic features. Non-HDL-c levels showed an increasing trend with age, from 118 (97–143) mg/dL in those <30 years old, 151 (124–175) mg/dL among those from 30–49 years old and 166 (137–196) mg/dL in >50 years old; p<0.001. On the other hand, indigenous Venezuelan populations showed lower non-HDL-c levels (127; 97–151 mg/dL) compared with mixed race (145; 116–175 mg/dL) and white Hispanics (145; 114–176 mg/dL; p<0.001).
| Non-HDL-C (mg/dL) | p* | |
|---|---|---|
| Median (p25–p75) | ||
| Age Groups (years) | <0.001 | |
| <30 | 118 (97–143) | |
| 30–49 | 151 (124–175) | |
| >50 | 166 (137–196) | |
| Ethnicity | <0.001 | |
| Mixed | 145 (116–175) | |
| Hyspanic white | 145 (114–176) | |
| Afro-venezuelans | 134 (108–164) | |
| Amerindians | 127 (97–151) | |
| Others | 147 (124–183) | |
| Alcohol consumption§ | 0.781 | |
| Yes | 142 (114–174) | |
| No | 144 (114–174) | |
| Tobacco smoke | <0.001 | |
| No smoker | 139 (110–169) | |
| Smoker | 151 (118–183) | |
| Former smoker | 150 (129–184) | |
| Physical activity (Leisure time dominion) | <0.001 | |
| Inactive | 147 (118–175) | |
| Very Low | 147 (117–178) | |
| Low | 140 (117–168) | |
| Moderate | 142 (111–180) | |
| High | 137 (112–174) | |
| Very High | 124 (98–160) |
Higher levels of non-HDL-c were found among smokers (151; 118–183 mg/dL) compared with non-smokers or ex-smokers, p=0.001. Subjects with very high physical activity exhibited lower non-HDL-c levels 124 (98–160) mg/dL when compared with sedentary subjects [147 (118–175) mg/dL; p<0.001]. No significant differences were found when comparing alcohol drinkers and non-drinkers.
Table 3 shows non-HDL-c levels according to clinical, metabolic, and anthropometric variables. Non-HDL-c were significantly higher among those with hypertension compared to those with normal blood pressure (159 vs. 132 mg/dL, respectively; p<0.001). This behavior was also observed when comparing obese and normal weight individuals (155 vs. 124 mg/dL, respectively; p<0.001), type 2 diabetes and non-diabetic individuals (161 vs. 137 mg/dL; p<0.001), abdominal obesity and persons with normal waist circumference (154 vs. 132 mg/dL; p<0.001), and elevated hs-CRP vs. normal hs-CRP (156 vs. 140 mg/dL; p<0.001). Tertile distribution according non-HDL-c and both, clinical and anthropometric variables are shown in Table 4.
| Non-HDL cholesterol | |||
|---|---|---|---|
| Median (p25–p75) | p* | ||
| BP JNC-7 | <0.001 | ||
| Normal blood pressure | 132 (106–158) | ||
| Pre-hypertension | 146 (118–175) | ||
| Hypertension | 159 (130–190) | ||
| BMI (kg/m2) | <0.001 | ||
| ≤24,9 | 124 (98–152) | ||
| 25–29,9 | 148 (119–180) | ||
| ≥30 | 155 (129–183) | ||
| Glycemic Status§ | <0.001 | ||
| Normo-glycemic | 137 (110–166) | ||
| Impaired Fasting Glucose | 155 (129–185) | ||
| DM2 | 161 (132–196) | ||
| Waist circumference† | <0.001 | ||
| Normal | 132 (105–161) | ||
| High | 154 (128–183) | ||
| hsCRP (mg/L) | <0.001 | ||
| <0,765 | 140 (109–169) | ||
| ≥0,765 | 156 (121–187) | ||
| Non HDL<130 | Non HDL=130–159 | Non HDL≥160 | χ2 (p) | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| BP JNC-7 | 101.58 (<0.001) | ||||||
| Normal blood pressure | 375 | 49.9 | 226 | 39.6 | 192 | 27.3 | |
| Pre-hypertension | 262 | 34.8 | 225 | 39.4 | 278 | 39.5 | |
| Hypertension | 115 | 15.3 | 120 | 21.0 | 233 | 33.2 | |
| BMI (kg/m2) | 151.54 (<0.001) | ||||||
| ≤24.9 | 343 | 45.6 | 159 | 27.8 | 120 | 17.0 | |
| 25–29.9 | 237 | 31.5 | 201 | 35.2 | 281 | 40.0 | |
| ≥30 | 172 | 22.9 | 211 | 37.0 | 302 | 43.0 | |
| Glycemic Status§ | 74.97 (<0.001) | ||||||
| Normo-glycemic | 609 | 81.0 | 408 | 71.5 | 426 | 60.6 | |
| Impaired Fasting Glucose | 102 | 13.6 | 116 | 20.3 | 186 | 26.5 | |
| DM2 | 41 | 5.5 | 47 | 8.2 | 91 | 12.9 | |
| Waist circumference† | 112.04 (<0.001) | ||||||
| Normal | 493 | 65.6 | 276 | 48.3 | 268 | 38.1 | |
| High | 259 | 34.4 | 295 | 51.7 | 435 | 61.9 | |
| hsCRP (mg/L) | 26.95 (<0.001) | ||||||
| <0.765 | 416 | 80.9 | 293 | 78.8 | 307 | 67.3 | |
| ≥0.765 | 98 | 19.1 | 79 | 21.2 | 149 | 32.7 | |
Figure 1 shows levels of non-HDL-c according to the number of risk factors for ASCVD. Those with any risk factor had a non-HDL-c of 122 (98–146) mg/dL, and 161 (131–192) mg/dL in those with three criteria and 159 (137–195) mg/dL in those with four criteria; p<0.001. Table 5 shows a multivariate logistic regression model where levels of non-HDL-c between 130 – 159 mg/dL, (OR=2.59; CI95%: 1.62-4.13; p<0.001) and ≥160 mg/dL (OR=3.75; CI95%=2.04-6.91; p<0.001), had an inverse probability of presenting a composite of multiple risk factors, while LDL-C was not significantly associated (OR=0.42; CI95%: 0.23-0,95; p=0.035).
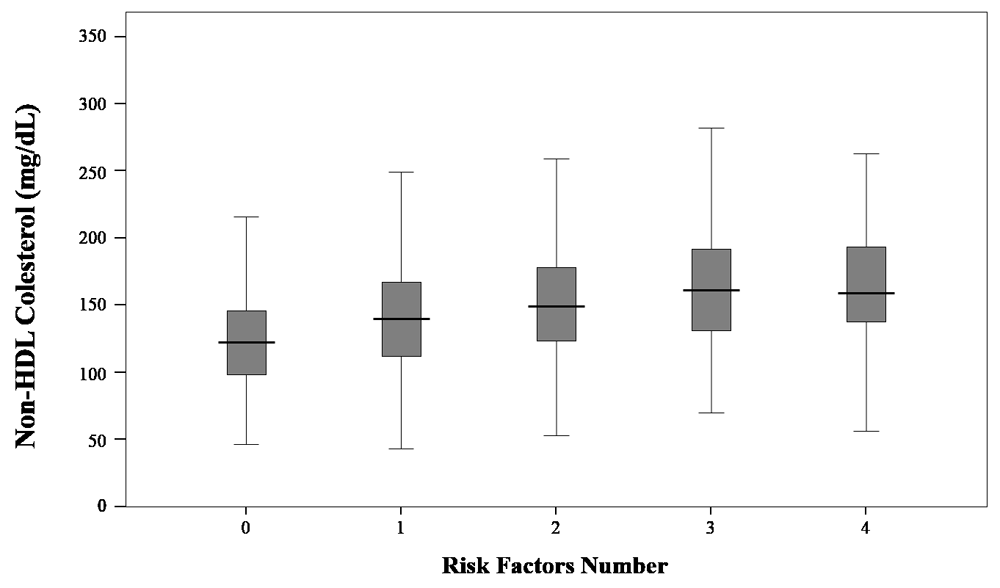
Kruskal-Wallis H Test: p<0.001.
| Dependent variable: MRFA (≥2 factors) | ||||
|---|---|---|---|---|
| Odds Ratio. crude (CI 95%a) | pb | Odds Ratio. adjustedc (CI 95%) | pb | |
| LDL-C | ||||
| <100 | 1.00 | - | 1.00 | - |
| 100–129 | 1.66 (1.33–2.08) | <0.001 | 0.75 (0.48–1.18) | 0.215 |
| ≥130 | 2.47 (1.99–3.08) | <0.001 | 0.42 (0.23–0.95) | 0.035 |
| Colesterol Non-HDL | ||||
| <130 | 1.00 | - | 1.00 | - |
| 130–159 | 2.08 (1.66–2.60) | <0.001 | 2.59 (1.62–4.13) | <0.001 |
| ≥160 | 3.65 (2.94–4.53) | <0.001 | 3.75 (2.04–6.91) | <0.001 |
For nearly 50 years, incredible efforts have been made to identify specific and prevalent ASCVD risk factors, planning and application of primary and secondary prevention strategies, evaluation of population genetics and overall ethnicity genetic risks, and modification due to epigenetics. These risk factors have been of various natures, from anthropometric measurements, such as BMI and waist circumference, lifestyle patterns, to blood lipids sub-fractions, such as LDL-c and HDL-c. In regards to the focus of the present study, lipid profiles and novel lipid fractions and their association with ASCVD have been the main focus of grand scale epidemiological, clinical, and pharmacological investigation31,32.
In spite of all the efforts, data has been accumulating that suggests that focusing on one lipid fraction, namely LDL-c, may not be the appropriate approach33, due to recently described atherogenic particles, like IDL, Apo B, and non-HDL33. The concept of cardiovascular residual risk factor has been intimately associated with cardiovascular disease reduction, being twice as effective as LDL-c34. In fact, Helgadottir et al.35 reported that genetic risk scores using non-HDL-c strongly associates with coronary artery disease, and this genetic risk was considerably lower than that offered by LDL-c. It is no coincidence that non-HDL-c has been shown to correlate with coronary artery disease progression, cardiovascular morbidity, and mortality34,36.
The present results show that higher non-HDL-c levels were associated with higher risk of multiple risk factors for ASCVD. These results are similar to those reported by Kumar et al. where non-HDL-c had a better predictive value than LDL-c for atherosclerosis among those with TGs >150 mg/dl37. This study excluded patients with increased TGs >400 mg/dl; therefore, one cannot assume this association is also seen in this group. Moreover, Arsenault et al.38 followed over 21 thousand subjects without diabetes or previous coronary heart disease (CHD), demonstrating that high non-HDL-c is associated with increased CHD.
Following the recommendation of the Strong Heart Study39, the recent 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk proposed a goal of <100 mg/dl for non-HDL-c in diabetic patients40. As expected, subjects with diabetes in our population have higher non-HDL-c, which is a recognized risk factor in diabetic subjects at risk for ASCVD41. Interestingly, Apo B and non-HDL-c are better predictors of diabetes development than glycated hemoglobin42. In line with this notion, the present results also show that non-HDL-c is associated with higher levels of hs-CRP (systemic inflammation), hypertension, and central obesity. We previously described our population as having a high prevalence of obesity and overweight, managing a staggering 65.7%43. Thus, the overlapping of risk factors and metabolic syndrome/type 2 diabetes development is imminent and borderline epidemic.
Lastly, Hispanic population seems to be at higher risk for LDL-particle numbers and non-HDL-c discordance44. Kilgore et al.45 reported that subjects with high non-HDL-c and normal LDL-c were likely to be Hispanic males with metabolic syndrome and other cardiovascular risks. Likewise, using the database from The Hispanic Community Health Study/Study of Latinos, Rodriguez et al.46 reported that almost two thirds of Latinos have a form of dyslipidemia, with South Americans having high non-HDL-c and high LDL-c. Therefore, ethnicity is of high importance when evaluating clinical risk for ASCVD, including blood lipid profiles and sedentary lifestyles in these groups47.
To summarize, this investigation in Hispanic population shows that non-HDL-c is associated with multiple risk aggregation for ASCVD, being associated with hypertension, central obesity and low grade inflammation. The question that arises is: Should non–HDL-c replace LDL-C as the main target of therapy?33. The fact that non–HDL-c is a better risk predictor, can be performed in a non-fasting state, and can be easily calculated by extracting HDL-c from total cholesterol without using any other laboratory assay makes it the most advantageous parameter for prediction of ASCVD even in subjects with TAG <200 mg/dl.
Dataset 1: MMSPS non-HDL and atherosclerotic cardiovascular disease risk factors raw data. DOI, 10.5256/f1000research.13005.d19598048
This work was supported by the Technological, Humanistic, and Scientific Development Council (Consejo de Desarrollo Científico, Humanístico y Tecnológico; CONDES), University of Zulia (grant nº CC-0437-10-21-09-10).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiovascular disease
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 19 Dec 19 |
||
|
Version 1 26 Apr 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)