Keywords
HIV, orthostatic hypotension, ANS impairments, blood pressure, postural instability, heart rate
HIV, orthostatic hypotension, ANS impairments, blood pressure, postural instability, heart rate
HIV can destroy or substantially affect essential cells (CD4+ T cells or T helper cells) of the immune system, which ordinarily help combat diseases. The virus spreads through the exchange of certain bodily fluids such as blood or as a result of unprotected sex. HIV affects more than 34 million people in the world according to WHO statistics, and more than 3.3 million North Americans aged 13 years and older, according to CDC statistics (Centers for Disease Control and Prevention, 2015). There is no cure for this disease, and if left untreated, it can progress to AIDS (acquired immune deficiency syndrome, defined as 200 or fewer CD4+ cells/µl); however, medication to control HIV exists (antiretroviral therapy) which can prolong the lives of patients (Song et al., 2017; WHO, 2011).
Patients with HIV can develop symptoms that vary depending on the disease stage. The most common symptoms are fever, fatigue, weight loss, swollen lymph nodes and shingles, amongst others. Typically, neurological complications, such as difficulty walking, are often seen in patients with advanced stages of HIV or AIDS. Nevertheless, there is one symptom that is not often mentioned, which it is known to affect HIV patients: orthostatic hypotension (OH) (Cohen et al., 1991; Robinson-Papp et al., 2013; Song et al., 2017; WHO, 2011).
By definition, OH is a reduction in blood pressure (BP) of at least 20 mmHg systolic or 10 mmHg diastolic after either 1 or 3 minutes of standing when the patient moves from supine or to the upright position. Symptoms of OH are often associated with medications, dehydration, and infections, as explained by Cohen et al., 1991. One theory of why OH may affect patients with HIV is due to impairments in the autonomic nervous system (ANS), which is typically responsible for the modulation of numerous functions, including heart rate and vasomotor tone (Robinson-Papp et al., 2013).
Although neurologic complications due to HIV are well characterized in the central and peripheral nervous systems, it is not entirely understood in the ANS due to its complexity. However, studies show that autonomic dysfunctions (Cohen et al., 1991) and autonomic neuropathy (Robinson-Papp et al., 2013) are common in patients with HIV. The symptoms of autonomic neuropathy, therefore, could include orthostatic dizziness or fainting, among others (Robinson-Papp et al., 2013).
Although there is some evidence of a connection between OH and impairments to the ANS, this connection is unclear and poorly understood. Therefore, the objective of this study is to evaluate OH during balance sensory condition tests (SCTs) and to assess the function of the cardiac autonomic system, such as BP and heart rate (HR), during a balance task. We hypothesized that BP and HR would rise with a change in postural positioning, thus revealing ANS dysfunction.
The study protocol was approved by the Institutional Review Board of the University of Puerto Rico Medical Sciences Campus (Protocol A2540114). Authorization was given by the Executive Director of the Institution. Every participant read and signed an informed consent document after being informed of all risks and discomforts related to their participation in this investigation.
We recruited eight Puerto Rican participants with a diagnosis of HIV from a community health center, La Perla de Gran Precio, in the San Juan area of Puerto Rico. The Physical Therapist from the Center identified possible participants for this study during May–July 2016, who then telephoned M.G.R. to confirm recruitment into the study. Subjects were of both sexes (4 males and 4 females), with age (±SD) =48.7±9.7 years, CD4 count =599.6±225.5 cells/µl, time of diagnosis =15.6±6.2 years and BMI of 21.8±2.8.
The study inclusion criteria were: i) HIV diagnosed by a medical doctor; ii) male or female; iii) aged 21–65 years old; and iv) CD4+ T cell count equal to or higher than 200. The exclusion criteria were: i) CD4+ T cell count lower than 200; ii) inability to walk; iii) severe balance impairment (≥2 falls within the last 6 months); iv) lower limb amputations; v) injury or surgery of lower limbs or back in the last 6 months; vi) lower limb ulcer(s) in the last 6 months; vii) pregnant women or women trying to get pregnant (women were asked if they were pregnant or there could be a chance there could be at the moment of the test); viii) any surgery in the past year; and ix) any hospitalization within the last year.
After providing written informed consent at the community-based fitness center in San Juan, Puerto Rico, anthropometric measurements were obtained from each participant. The anthropometric measures included height and weight which were measured with a scale and stadiometer. Following the anthropometric measures (height and weight), a sensibility assessment was performed with a monofilament on both feet.
In addition to the exclusion and inclusion criteria aforementioned, these too will serve as a more accurate criterion for the study. The screening assessments are described as follows:
Participants with a body mass index (BMI) of 18.5 or below (underweight) and 30.0 and above (obese) were excluded from the study. Thus, participants were required to have a BMI of 18.5–29.9.
A Semmes-Weinstein monofilament was used to evaluate the sensation threshold on the foot area. The monofilament was applied perpendicular to the skin's surface. Neuropathy can be detected using the 5.07 monofilament (this filament bends with the application of a 10-g force). Participants unable to detect the 5.07 monofilament (loss of protective sensation and deep pressure sensation) in more than two areas were excluded from the study (Bell-Krotoski et al., 1993)
An iHealth Wireless Blood Pressure Monitor (iHealth Model: BP5) (a wireless BP monitor for the arm) was used to measure systolic BP (SBP) and diastolic BP (DBP), and heart rate.
SBP, DBP and heart rate (HR) were measured after 5 minutes of sitting, immediately after standing up on the floor (firm surface), and 1 minute after beginning the SCT. In the SCT, patients were asked to close their eyes and actively nod their head up and down while standing on foam (an unstable surface), which eliminated visual and proprioceptive input and stimulated the vestibular system. The participants were instructed, while standing on foam, to sustain a set frequency (approximately one turn per second with a metronome, 60 bpm) and amplitude (about 45 degrees in each direction for flexion and extension) of movement to ensure the average velocity of movement was maintained. Once the participants got the rhythm, they were instructed to close their eyes for 30 seconds.
The comparisons in this study were as follows: First, we compared BP and HR during the sitting position and standing position. Secondly, we compared, BP and HR during the sitting position with standing position during the SCT. Lastly, we examined BP and HR in subjects while they were standing during the SCT.
Statistical analysis was performed using SPSS version 20 software package for Windows. A paired sample Student’s t-test was used to assess the difference between BP sitting and BP standing, and standing versus standing during the SCT. HR was also assessed the same way. We considered a P-value of 0.01 as significant.
We analyzed eight subjects of different genders and years of HIV diagnosis from a health center in San Juan, Puerto Rico (Table 1). Raw data of heart rate, SBP and DBP is reported in Table 2. The average of BP and HR measurements for each subject before and after standing to observe possible ANS deficits associated with HIV (Table 2–Table 3).
We assessed HR (Figure 1), and SBP (Figure 2) and DSB (Figure 3) to determine whether there were ANS alterations in these participants.
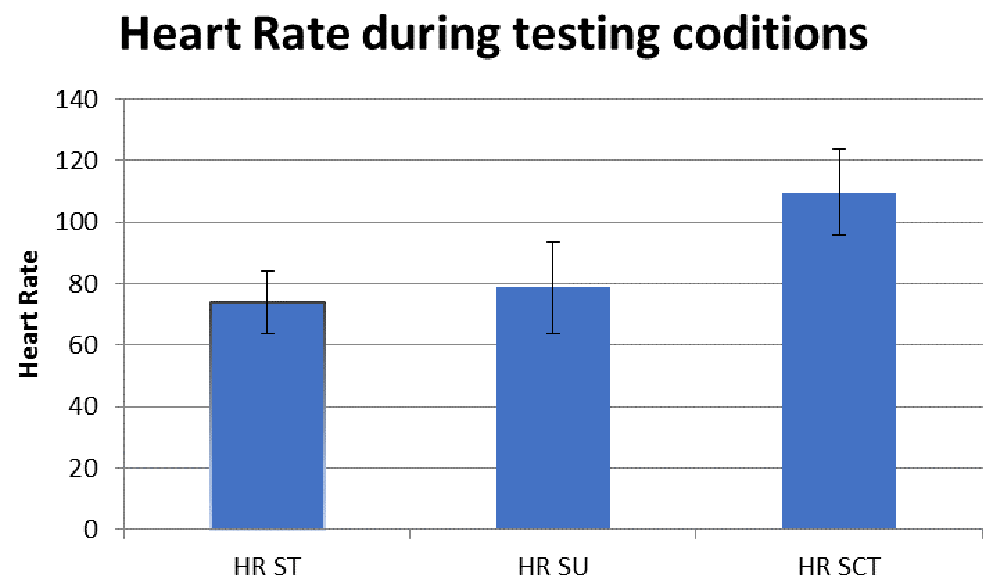
ST, sitting; SU, standing; SCT, standing while performing the sensory condition test.
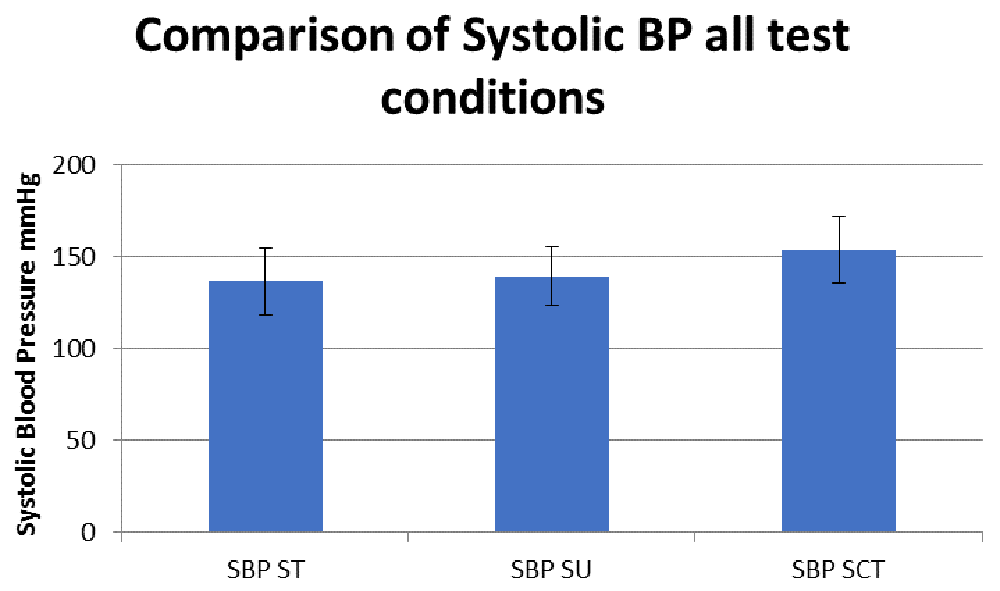
ST, sitting; SU, standing; SCT, standing while performing the sensory condition test.
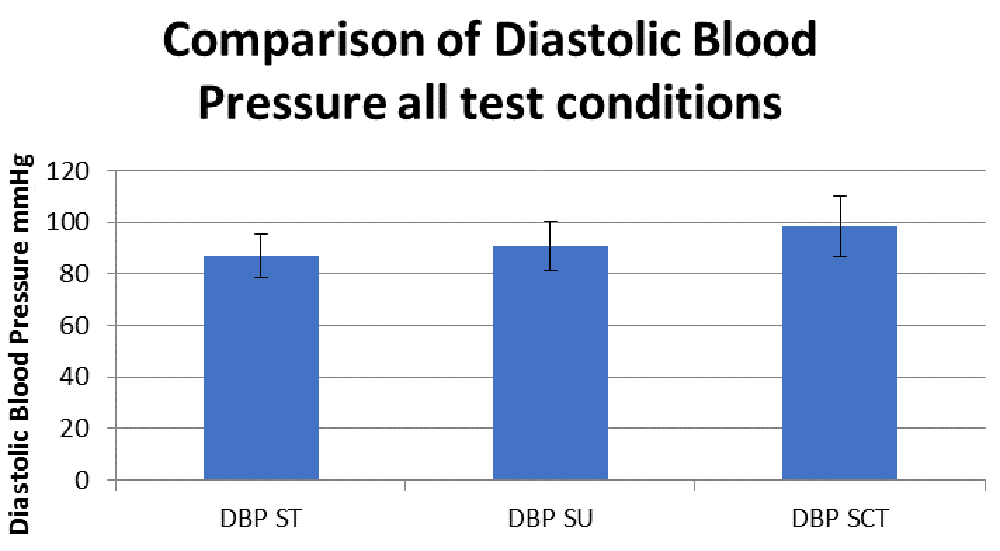
ST, sitting; SU, standing; SCT, standing while performing the sensory condition test.
All comparison are reported in Table 4. Results revealed no difference in BP and HR in sitting versus standing. However, there was a significant increase in SBP (mean ± SD) (SP, 136.5±17.8; BT, 153.7±16.5; P<0.01) and HR (SP, 73.9±10.1; BT, 109.7±13.9; P<0.05) when subjects changed positions from standing to standing during the SCT (see Figure 2). Additionally, in our last comparison, standing versus standing during the SCT, there was a significant increase in SBP (SP, 139.4±16.2; BT, 153.8±16.5; P<0.01) and HR (SP, 78.9±14.7; BT, 109.7±1.9; P<0.05).
We hypothesized that BP and heart rate would increase with changes in postural positioning, thus revealing ANS dysfunction. The key findings of our study were that participants demonstrated an increase in SBP and HR when going from sitting to standing while performing the SCT. There was also an increase in SBP and HR from standing to standing while carrying out the SCT. Therefore, our hypothesis was accepted. The present study provides support for the presence of autonomic dysfunction in persons with HIV.
The fact that SBP and HR changed from sitting and standing (on foam) could, by themselves, be causing alterations in the cardiovascular system. When we stand, muscles in charge of venous return in the lower extremities are activated (Recek, 2013). It is possible that muscle weakness is responsible for improper venous return, thus causing an increase in HR as a compensatory mechanism (Dymarek et al., 2014). However, the fact that BP and HR changed from standing to standing while performing the SCT was surprising. Head movements during the SCT could be affecting the carotid body (baroreceptor), thus resulting in fluctuation of BP and HR (Ogoh et al., 2003). However, it is important to point out that normal head tilts or movements do not cause BP changes (Ogoh et al., 2006) like the ones observed in the present study of subjects with HIV.
Venous return can also be influenced by the difficulty experienced by individuals standing on a foam/complaint surface or their inability to do so. Lower extremities muscles will co-contract, thus increasing muscle activity to maintain the standing position. (Shumway-Cook & Woollacott, 2007).
Previous studies suggest that ANS alterations in patients with HIV are linked to the use of medications to control the HIV virus itself (Cohen et al., 1991). Other studies also show that there are neurologic deficits in people with HIV, specifically with the ANS. Although the ANS regulates many different bodily functions, we were most interested in identifying alterations within the ANS during balance tests and the cardiac autonomic response (BP and HR) during the same task. However, there is a lack of evidence concerning why OH affects many people with HIV.
An initial study by Cohen et al. in 1991 suggests that patients with HIV will have OH as a feature of ANS impairments. An update is needed to address the recent data related to OH and HIV. Therefore, we studied BP and HR in patients with HIV to reveal OH alteration. In our initial results, there was an increase in BP when participants were asked to go from a sitting to standing position, albeit not a significant one. It is possible that with these changes in BP and HR our participants with HIV are exhibiting ANS alterations or dysfunctions. Despite the different methodology used in this study, our results had similar findings compared to the information available in the literature (Cohen et al., 1991).
In this study, there was a significant increase (p<0.01) in HR when standing compared to HR when standing and performing the SCT. These results were surprising because such values have never been reported before. This study shows that the ANS may be impaired in people with HIV; however, more research is needed to confirm these findings.
Some of the clinical implications of this study indicate the need for further investigation of the neuromuscular activation of lower extremity muscles responsible for antero-posterior sway (tibialis anterior/gastrocnemius) and venous return (gastrocnemius). Additionally, peripheral neuropathy testing should be considered when evaluating ANS changes in persons with HIV. Towards identifying ANS dysfunctions in this population, clinicians should observe changes in HR and BP during functional movements, such as moving from a supine position to sitting, and from sitting to standing.
All data underlying the results are available as part of the article and no additional source data are required.
We would like to thank ‘La Perla de Gran Precio' for their constant support and to the patients who were willing to participate in this study.
This article was published with support from Texas Woman's University Libraries’ Open Access Fund.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 04 Jun 18 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)