Keywords
Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, cirrhosis, fibrosis
Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, cirrhosis, fibrosis
Non-alcoholic fatty liver disease (NAFLD) is the condition in which hepatic fat accumulation is present after all other causes of hepatic steatosis are excluded; these include liver disease caused by other factors, excessive alcohol consumption, and other conditions that may lead to hepatic steatosis. The clinical spectrum of NAFLD is wide-ranging and spans NAFL to non-alcoholic steatohepatitis (NASH), advanced fibrosis, cirrhosis, and hepatocellular carcinoma (HCC). NAFLD is the most common liver disease in the world, and NASH may soon become the most common indication for liver transplantation1. The incidence and prevalence of NAFLD is rising globally owing to increasing rates of obesity and diabetes2,3. The development of liver cancer in patients with NAFLD, even without the presence of cirrhosis, was observed recently in a number of studies. These outcomes contribute substantially to the burden of disease to the individual and also to society. It is therefore a public health priority to develop effective measures for the identification and treatment of this condition. The recent advances in understanding and managing NAFLD are reviewed below.
The true worldwide incidence rate of NAFLD/NASH is not known. It was found through a few studies that the incidence rates were widely variable owing to multiple factors which include the different characteristics of study populations, exclusion criteria, study methodology, and approach for diagnosis. The variable presentations of the disease as well as the unavailability of sensitive diagnostic studies besides the liver biopsy, which remains the gold standard to date, probably contribute to the underreported incidence and prevalence of NAFLD4. Analysis of liver ultrasound data collected between 1988 and 1994 from the Third National Health and Nutrition Examination Survey (NHANES III) reported that 19% of adults have NAFLD, while a meta‐analysis of studies from 2006–2014 estimated a NAFLD prevalence of 24% (20–29%) in the general population. The Dionysos5 study in Italy first reported that the global prevalence of NAFLD is 24–25% of the general population; this is the most accurate estimated figure to date, and it was confirmed recently by Younossi6,7, who described some regional differences with the highest rates reported in South America and the Middle East, followed by Asia, the USA, and Europe. The increasing prevalence of NAFLD/NASH is in parallel to the pandemic spread of obesity, diabetes mellitus (DM), and metabolic syndrome. One-third of American adults are thought to have NAFLD8. The prevalence of NAFLD in Europe and the Middle East ranges from 20–30%. The highest prevalence of NAFLD was reported in South America and the Middle East, whereas the lowest was reported in Africa. Although the global burden of NAFLD is unknown, on the basis of the above studies, we estimate that ~one billion individuals have NAFLD at the current time. Liver biopsy is the gold-standard diagnostic test. There are also some non-invasive diagnostic modalities including hepatic ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). The discrepancy in prevalence data for NAFLD could be attributed to the difference in sensitivity of these diagnostic tests. Younossi et al. reported that the pooled regional NAFLD prevalence estimates among patients diagnosed by blood test were 13.00% (95% confidence interval [CI]: 4.44–32.47) for Europe, 12.89% (95% CI: 8.32–19.44) for North America, and 9.26% (95% CI: 7.07–12.05) for Asia9. Estes et al. reported that by 2030, the NAFLD population was projected to increase by 21% to 100.9 million cases. Prevalence in 2030 is estimated at 33.5% (aged ≥15 years) and 28.4% (all ages). The NAFLD population is estimated to have a median age of 50 (2015), which increases to 55 by 2030. During 2015–2030, there would be 1.2 prevalent NAFLD cases among male individuals for every 1.0 among female individuals10. Because of the small number of studies that contained NAFLD incidence results, Younossi et al.’s meta-analysis results were obtained only for Asia (only available for China and Japan) and Israel. In Asia, the pooled regional NAFLD incidence rate was estimated to be 52.34 per 1,000 person-years (95% CI: 28.31–96.77), and in Israel it was estimated to be 28.01 per 1,000 person-years (95% CI: 19.34–40.57)9. According to Estes et al., the fastest growth in obesity prevalence occurred during 2000–2002. In comparison, the fastest growth in DM prevalence occurred during 2012–2014. The number of NAFLD cases increased following obesity, resulting in a peak NAFLD incidence in 2008 with an estimated 4.17 million new cases. Since then, a slowing rate of increase in NAFLD was forecasted, and new cases were estimated to decline to 3.62 million annually. Thus, the total number of NAFLD cases is still increasing but at a lower rate compared to the 2005–2008 period. However, given the rising prevalence of type 2 diabetes, the proportion of individuals with NASH is predicted to increase, and with prolonged disease exposure the number of individuals with end-stage liver disease is likely to triple by 2030. It has been estimated that, in the USA, NAFLD is responsible for $292 billion in annual medical and societal costs10. The projected cost of caring for patients is expected to increase by 18% from 2000 to 2035, and the health-related quality of life of NAFLD patients is described as declining11,12.
A substantial body of literature has accumulated and provides a framework to understand the pathogenesis of NAFLD. With increasing caloric intake and changes in dietary composition, excess calories are stored as fat within adipose tissue and also induce changes in the microbiome. This triggers changes in intestinal permeability and increased systemic exposure to intestinal microbial products, triggering activation of the innate immune system and adipose tissue inflammation. The metabolic consequence is the development of an insulin-resistant state. The insulin-resistant state which drives increased lipolysis along with the consumption of excess calories deliver an increased load of lipotoxic lipids including free fatty acids to the liver along with excess carbohydrates. This is further compounded by increased de novo lipogenesis, which is driven by hyperinsulinemia and retained sensitivity to the lipogenic effects of insulin in an otherwise insulin-resistant state. The liver attempts to react by increasing lipid oxidation and export of lipids; when lipid influx and synthesis exceed its metabolism and export, the excess lipids accumulate in lipid droplets, creating a fatty liver. Recently, it has been shown that the PNPLA3 protein accumulates on the surface of lipid droplets13. Under conditions of lipotoxic stress, proteasomal impairment drives such accumulation where, in those with mutant PNPLA3, impaired lipolysis leads to further accumulation of fat. However, this does not explain how the mutation drives the development of steatohepatitis and cirrhosis. Cell stress including oxidative stress and unfolded protein response can trigger apoptosis, cell death, and inflammation. Apoptosis can also trigger cell regenerative activity. While much is known about how lipotoxicity drives cell death and inflammation, there is still a paucity of information on the biological mechanisms driving tissue adaptation and regeneration. Prolonged inflammation drives fibrogenic remodeling of the liver. Recently, considerable advances in the development of in vivo and in vitro models of NASH have been made14–16. The criteria for the validation of such models as models of human NASH are clearer17. In reviewing data from such models for understanding human disease, it is important to ascertain if such validations have been performed in the model. These criteria include whether gene manipulations that are not reflective of the human state are used, if the macronutrient composition reflects human diet, if features commonly seen in humans such as obesity, systemic inflammation, dyslipidemia, insulin resistance, steatohepatitis, and fibrosis are present, and if there is concordance of cell signaling and transcriptome to human disease.
Most of the data on the natural history of NAFLD are based on indirect evidence and have come from a selected population. Despite NAFLD’s high prevalence, only a minority of NAFLD patients progress to significant fibrosis or experience associated morbidity18. The reason for this variability is, partly, subtle individual genetic differences that change one’s response to environmental factors and lifestyle, thus determining disease phenotype19–21. Recent data from a population study in Olmsted County reported a significantly higher mortality in NAFLD patients compared with the general population; however, overall mortality and liver-related death were lower than previously reported from referral centers. Mortality in these patients was associated with older age, glucose intolerance/diabetes, and the presence of cirrhosis. The top three leading causes of death in patients with NAFLD in descending order are cardiovascular disease, cancer, and liver disease22. A patient’s chance of progressing to advanced liver disease, including hepatic decompensation and HCC, is higher if they suffer from NASH than if they suffer from NAFLD. Recent studies have suggested that NAFLD can evolve to NASH with advanced fibrosis, which would imply that it may not be an entirely benign condition23,24. A recent study found that 44% of patients with NAFLD at the index liver biopsy progressed to NASH and 37% progressed to fibrosis, including 22% to advanced degree25. Managing NAFLD requires markedly increased healthcare resources as fibrosis worsens, especially after the development of cirrhosis. Although there are no exact models to estimate the incidence and the disease burden of NAFLD in the next few years, the changing trends of obesity and DM suggest that this problem is increasing worldwide and might place a growing strain on healthcare systems.
NAFLD remains asymptomatic in a significant proportion of patients, and the diagnosis is often suspected when liver functions are found to be abnormal on biochemical testing or hepatic imaging (ultrasonography, CT, or MRI of liver) suggests fatty liver when performed for some other reason. Liver biopsy remains the gold standard for diagnostic evaluation of NAFLD. In the past, the NASH Clinical Research Network histological scoring system was the most widely used, representing a validated scoring system that generates a NAFLD activity score (NAS). A NAS of 5 or more is sometimes considered NASH and less than 3 is considered not NASH26. However, NAS cannot be used as a surrogate for discrimination between NASH and NAFLD, although it is useful for the histological diagnosis27,28. Given that the prevalence of NAFLD is high, using liver biopsy to detect fibrosis-cirrhosis is unfeasible. The accuracy of liver biopsy to assess fibrosis has also been questioned owing to sampling errors and intra- and inter-observer variability that may lead to over- or under-staging. Cost, procedure-related complications, and intra- and inter-observer variations in reporting the histology are the major drawbacks of liver biopsy, and, therefore, it is usually not recommended in clinical practice, except in circumstances where other differential diagnoses are to be excluded. Over the past decade, there has been a growing interest in alternative novel strategies for the non-invasive evaluation of fibrosis. These techniques require two distinct but complementary approaches: the measurement of serum biomarkers or the estimation of liver stiffness using ultrasound-based elastography with transient elastography (TE) as the forerunner29. In NAFLD patients, TE, the fibrosis index (FIB-4), and NAFLD fibrosis scores are the best validated non-invasive tests30,31. For instance, in a recent meta-analysis32 based on 64 studies including a total of 13,046 NAFLD patients, the summary AUROC values of TE, FIB-4, and the NAFLD fibrosis score for diagnosing severe fibrosis-cirrhosis were 0.88, 0.84, and 0.84, respectively. When all three were compared head to head, TE was the most accurate in the diagnosis of cirrhosis33. Lastly, recent evidence shows that non-invasive tests, including FIB-4 and NAFLD fibrosis score as well as TE-derived liver stiffness measurements, accurately identify the subgroup of patients with NAFLD at a higher risk of developing liver-related complications and death or liver transplantation34. Liver enzymes can often be normal in a number of patients with NAFLD. Although several biochemical markers, such as TNF-a, IL-6, CRP, pentraxin, ferritin, serum prolidase enzyme activity, soluble receptor for advanced glycation end product, and cytokeratin-18, have been proposed as useful in predicting the severity of NAFLD/NASH in the past, none of these markers have shown sufficient sensitivity or specificity for routine clinical application for diagnosis35. Ultrasonography, CT, and MRI of the liver are the standard imaging modalities used in clinical practice for the diagnosis of NAFLD. In general, about 30% of liver steatosis cases should be present for sonography to detect NAFLD. TE is an ultrasound-based imaging technique to detect the degree of fibrosis in patients with NAFLD and NASH. The sensitivity and specificity of TE to diagnose various stages of fibrosis have been reported to be 79–92% and 75–92%, respectively36. Recent evidence also suggests that the ultrasound-based controlled attenuation parameter value used in the TE technique can predict the degree of steatosis in patients with NAFLD37. The gold standard for the non-invasive assessment of hepatic steatosis is the use of MRI protein density fat fraction. Newer MRI techniques, such as MR elastography, can stage the degree of fibrosis non-invasively to diagnose and assess the prognosis of patients with NAFLD38.
Lifestyle modification, consisting of diet and exercise, is the cornerstone of therapy for NAFLD and has been shown by many studies to improve liver histology39,40. However, lifestyle modification is difficult to achieve and to sustain41. The remarkable progress that has been made in previous years in understanding disease pathogenesis has led to an explosion of medical therapies targeting various aspects of the fat accumulation and injury pathways. These therapies can be classified according to their intended targets into four general groups (Figure 1).
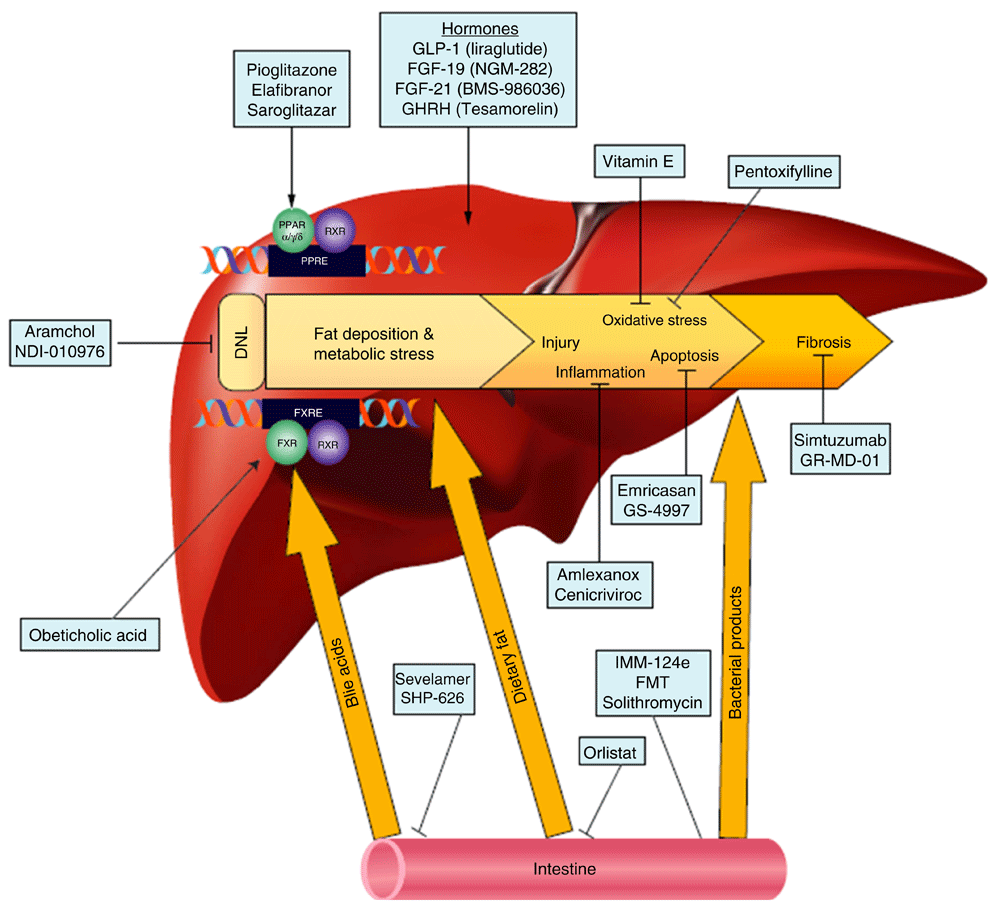
DNL, de novo lipogenesis; FGF, fibroblast growth factor; FMT, fecal microbial transplant; FXR, farnesoid X receptor; FXRE, FXR response element; GHRH, growth hormone-releasing hormone; GLP-1, glucagon-like peptide-1; PPAR, peroxisome proliferator-activated receptor; PPRE, PPAR response element; RXR, retinoid X receptor. Reprinted with permission from Rotman Y and Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–190.
Antioxidants. Large randomized clinical trials have proven the beneficial effects of vitamin E in patients with NASH (Table 1). In an innovative randomized double-blind placebo-controlled trial (RDBPCT), the authors found that a daily dose of 800 IU of vitamin E for 96 weeks improved the histological features of NASH (hepatic steatosis, lobular inflammation, and hepatocellular ballooning) in approximately 43% of non-diabetic patients compared with 19% of placebo (P = 0.001)42. In an analysis conducted recently of patients from the control group of the FLINT trial, vitamin E’s ability to improve NASH histological features was further confirmed43,44.
ACC, acetyl-CoA carboxylase; ASBT, apical sodium-dependent bile acid transporter; ASK1, apoptosis signal-regulating kinase 1; CCR, C-C chemokine receptor; GLP, glucagon-like peptide; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GHRH, growth hormone-releasing hormone; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; LOXL2, lysyl oxidase-like 2; miR, microRNA; PDE, phosphodiesterase; PPAR, peroxisome proliferator-activator receptor; SCD, stearoyl CoA desaturase; TBK1, TANK-binding kinase 1; VAP, vascular adhesion protein.
Lipid-lowering agents. A recent study demonstrated the underutilization of statins in patients with NAFLD45 (Table 1). A prospective trial in 20 patients with biopsy-proven NASH and dyslipidemia determined the effect of 12 months of rosuvastatin (10 mg/day) on liver histology. In total, 19 out of the 20 patients enrolled demonstrated complete resolution of NASH despite no change in weight compared with baseline46. Aramchol is a synthetic lipid that inhibits the stearoyl coenzyme A desaturase 1, a key enzyme in lipid metabolism47. A recent phase II RDBPCT compared two doses of aramchol (100 mg and 300 mg, daily) to placebo for three months in 60 patients with biopsy-proven NAFLD including six with NASH. At the end of the trial, hepatic fat was significantly reduced, as measured by magnetic resonance spectroscopy, in patients who were treated with 100 mg daily aramchol compared to those given placebo (12.57% versus 6.39%, respectively; P = 0.02)48.
Targeting insulin resistance
Bile-acid-based insulin sensitization
Farnesoid X receptor (FXR) is a nuclear bile acid receptor which inhibits further bile acid production when activated via the rate-limiting enzyme cholesterol 7 alpha-hydroxylase (CYP7A1) (Table 1). A recent study demonstrated that there are changes in the bile acid composition in those with NASH49. There is a progressive increase in primary bile acids and a decrease in secondary bile acids, which would be predicted to reduce FXR effects.
Obeticholic acid (OCA) is a selective FXR agonist that showed promising anti-inflammatory and anti-fibrotic effects in animal studies50. Its potential has been realized in the FLINT trial, where it was shown to be superior to placebo for the reduction of disease activity as well as fibrosis. It also increases LDL cholesterol and causes pruritus in up to 20% of individuals. This has led to efforts to develop small molecules as FXR agonists that do not have these adverse effects. Several such compounds are in early phase trials. It is also proposed that binding of FXR by bile acids in the intestine releases FGF19, which also ameliorates NASH; a recent early phase trial demonstrated rapid de-fatting of the liver with FGF19. On the other hand, blocking bile acid re-absorption in the ileum also depletes the bile acid pool and pulls cholesterol into bile acid synthesis, decreasing the cholesterol load. Further delivery of bile acids to the ileum can release GLP1, which has insulin-sensitizing properties. The use of bile acid transport inhibitors is being tested in clinical trials.
Recently, a number of additional bile acid receptors have been proposed as targets for drug development in NASH. TGR5 is a receptor with important functions and effects on hepatic lipid regulation and glucose metabolism. INT-767 is an investigation drug which activates both FXR and TGR5 and is being studied currently in phase IIb RDBPCT in patients with NASH (NCT02854605).
Peroxisome proliferator-activator receptors. Recently, a peroxisome proliferator-activated receptor (PPAR) δ agonist, MBX-8025, was shown to abolish lipotoxicity and ameliorate NASH in a diabetic mouse model51. Elafibranor (GFT-505) is a dual PPARα/δ agonist which has been shown to improve liver, adipose tissue, and peripheral tissue insulin sensitivity and reduce alanine aminotransferase (ALT) levels in patients with metabolic syndrome52. Thiazolidinediones, including pioglitazone, are PPARγ agonists used in the treatment of diabetes and have been demonstrated to be effective in NASH53.
The glitazars are dual PPARα/γ agonists which aim to combine the beneficial effects of activating both PPAR receptors. In a mouse model of NASH, saroglitazar was found to reduce steatosis and ALT as well as improve liver histology54. A subsequent retrospective study of NAFLD patients with dyslipidemia treated with saroglitazar for 24 weeks showed a significant decrease in ALT compared with baseline55. A phase II open-label study (PRESS VIII) evaluated the effectiveness of saroglitazar among 32 patients with biopsy-proven NASH54. After 12 weeks of treatment, a 52% decrease in ALT was shown. A phase III RDBPCT is currently ongoing in India to assess the effect of saroglitazar versus placebo for 52 weeks in biopsy-proven non-cirrhotic NASH (Clinical Trials Registry-India CTRI/2015/10/006236) (Table 1).
Metformin. A recent meta-analysis has shown that metformin leads to normalization of serum aminotransferases in a significantly greater proportion of patients when compared to dietary changes, and it also improved steatosis on imaging56.
Incretins and sodium–glucose cotransporter 2 inhibitors. Recently, a multicenter RDBPCT evaluated liraglutide in 52 subjects with NASH. After 48 weeks of treatment, NASH resolved in 39% of patients treated with subcutaneous liraglutide injections compared to only 9% in the placebo group (relative risk 4.3 [95% CI: 1.0–17.7]; P = 0.019). Additionally, two patients on liraglutide (9%) versus eight patients (36%) on placebo exhibited fibrosis progression57. The newest class of diabetic medications on the market are sodium–glucose cotransporter 2 (SGLT-2) inhibitors. A recent retrospective study evaluated the effectiveness of SGLT-2 inhibitor ipragliflozin (50 mg/day) on liver enzymes and FIB-4 in 50 diabetic NAFLD patients58. Over an average follow-up of 451 days, there was a significant decrease in body weight, ALT, and FIB-4 compared with baseline values (Table 1).
FGF21. This is a hepatokine with insulin-sensitizing and anti-fibrotic properties. In a phase IIa study, daily as well as weekly dosing of FGF21 was associated with improvement in hepatic steatosis and a decrease in hepatic stiffness.
Cenicriviroc (CVC) is an oral dual antagonist of C-C chemokine receptors (CCR2/CCR5) that was recently studied in a phase IIb RDBPCT, the CENTAUR trial. The trial showed improvement of fibrosis by at least one stage (P = 0.023) and a decrease in IL-6, high-sensitivity CRP, and fibrinogen levels in patients treated with CVC compared to placebo. A phase IIa study of cenicriviroc (ORION) aiming to assess the effect of 24 weeks of treatment on insulin sensitivity, liver enzymes, and liver imaging in obese patients with insulin resistance and suspected NAFLD in HIV-infected subjects provided encouraging initial data (NCT02330549). The CENTAUR study by Friedman et al. evaluated the effect of cenicriviroc on the histology of NASH59; this showed improved biochemical evidence of inflammation and histological evidence of decreased fibrosis. However, it did not affect the presence of steatohepatitis or disease activity as assessed by histological assessment.
Another investigational anti-inflammatory agent is amlexanox; it functions through different mechanisms by inhibiting NFκB-pathway-produced IKKε kinases and TANK-binding kinase 1 (TBK1). This drug, currently used for the treatment of recurrent aphthous ulcers and asthma, is being studied in a phase II RDBPCT in obese patients with type 2 diabetes and NAFLD (NCT01975935) (Table 1).
Emricasan is a first-in-class caspase protease inhibitor that has been studied in preclinical settings. In a recent phase II RDBPCT of 38 study participants with non-cirrhotic NAFLD, 28 days of emricasan (25 mg twice daily) resulted in a substantial decrease in liver enzymes and cytokeratin 18 fragments, a surrogate of liver apoptosis60. A phase IIb trial of emricasan versus placebo (ENCORE-NF) is currently ongoing to evaluate the efficacy of 72 weeks of emricasan (10 mg per day or 100 mg per day) in patients with biopsy-proven NASH. The primary outcome is improvement in fibrosis without worsening of NASH (NCT02686762). Inhibition of apoptosis signal-regulating kinase (ASK1) reduces liver steatosis and fibrosis in a murine model of diet-induced NASH61. An open-label phase II trial in 72 NASH patients with stage 2/3 fibrosis randomized to an oral ASK1 inhibitor, selonsertib (formerly called GS-4997; 6 mg or 18 mg/day), versus lysyl oxidase-like 2 antibody simtuzumab (25 mg subcutaneous weekly) versus selonsertib and simtuzumab was recently completed62. Preliminary analysis showed that patients who received selonsertib (with or without simtuzumab) were more likely to demonstrate decreased hepatic steatosis, decreased fibrotic stage, and at least 15% decrease in liver stiffness on magnetic resonance elastography compared with simtuzumab alone. Anti-steatotic and anti-fibrotic effects of selonsertib were dose dependent63 (Table 1).
The lysyl oxidase-like 2 antibody simtuzumab is currently in a phase IIb trial in NASH patients with advanced fibrosis but without cirrhosis (NCT01672866) (Table 1). Study participants are randomized to biweekly subcutaneous injections of simtuzumab (75 or 120 mg) versus placebo for 96 weeks, followed by an additional 240 weeks of open-label phase. Simtuzumab is also being investigated in a phase IIb trial in NASH patients with compensated cirrhosis (NCT01672879). The primary end point being assessed is mean change in hepatic venous pressure gradient as well as event-free survival. Galectin-3 is a member of a family of proteins that bind to terminal galactose residues on glycoprotein and is found to be increased during acute or chronic inflammation resulting in fibrogenesis. GR-MD-02 is a galectin-3 inhibitor that was shown to improve NASH fibrosis in animal studies63 and is now being evaluated in two phase II RDBPCTs in patients with NASH fibrosis and NASH cirrhosis, one of which has already completed enrollment (NCT02462967, NCT02421094). The final results of a phase II trial indicated that a subset of individuals with mild portal hypertension improved on galectin.
Microbiome-based therapies. Bovine colostrum is enriched with IgG directed against antigens injected into cows immediately prior to calving. An IgG-rich bovine colostrum extract, IMM-124e, generated from cows immunized against lipopolysaccharide was shown to improve insulin sensitivity, glycemic control, and liver enzymes in a small pilot study64. A phase II trial is currently evaluating 24 weeks of IMM-124e on biopsy-proven NASH (ClinicalTrials.gov identifier NCT02316717). Solithromycin, a macrolide antibiotic with anti-inflammatory properties, has been found to improve NASH in animal studies65 and is currently being studied in a phase II clinical trial (NCT02510599) (Table 1).
NAFLD is the most common cause of chronic liver disease in the Western world today. With rising levels of obesity and type 2 DM, its prevalence will increase in the future and cause considerable morbidity and mortality. The field of NAFLD continues to evolve rapidly. Advances in the understanding of classical steatosis and disease progression are also reviewed with a view toward providing translational insights into how this knowledge can be used to prevent or treat the disease in the future. Despite considerable research and multiple clinical trials, at present no single pharmacologic agent has achieved a clinically meaningful benefit/risk profile to warrant regulatory approval for marketing. There are currently a number of drugs undergoing pivotal trials as potential therapy for NASH. It is anticipated that the first drugs to be approved for NASH will likely become available by 2020. The ideal treatment will lead, in the short term, to a reduction in liver inflammation and fibrosis as well as an improvement in insulin sensitivity and metabolic complications but, in the long term, will need to reduce cardiovascular and liver outcomes.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 Jun 18 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)