Keywords
polyvinyl chloride (PVC), Pluronic F127, pore forming agent
polyvinyl chloride (PVC), Pluronic F127, pore forming agent
This is the revised manuscript based on the referee comment. The changes are:
See the authors' detailed response to the review by Heba Abdallah
See the authors' detailed response to the review by Zulfan Adi Putra
Nowadays, separation of contaminant elements from drinking water using membrane technology is developing rapidly. Membrane separation technology has been adopted in many industries, owing to its numerous advantages compared with other common methods. One of the most widely applicable membrane separation technologies in industry is the use of a group of ultrafiltration (UF) membranes, particularly for the process of water purification1,2. In view of the requirements for application in the water treatment industry, the modification of UF membrane to generate high flux, improve the resistance to fouling and chemical substances, and provide good mechanical properties is being continuously improved3,4.
Polyvinyl chloride (PVC), with the molecular formula shown in Figure 1, is a relatively cheap polymer with suitable chemical characteristics for use as a membrane material. Hydrophobic PVC polymers cause fouling of the membrane pores due to the adherence of organic molecules to the surface of the membrane. Numerous methods have been developed to improve fouling resistance. The most common method is improving the hydrophilicity of the membrane material to minimize the attachment of foulant molecules5,6.
The hydrophilic polymers that are frequently used as an additive are polyvinylpyrrolidone, polyethylene glycol, Brij, Tetronic, and Pluronic7,8. Of these, Pluronic is used as a surface modifying agent for many hydrophobic polymers. Raslan and Mohammad9 added Pluronic F127 to a polysulfone membrane. The resulting membrane is resistant to fouling and possesses good pore distribution9. Pluronic has also been used to improve the anti-fouling of cellulose acetate (CA) membranes10. The combination of CA polymer and Pluronic surfactant results in a membrane that is more resistant to fouling and has a more stable filtration profile. Another study investigate the hydrophilic surfactant Tween20 and Tween80 to enhance the permeation and antifouling properties of PVC membrane11. The surfactants were added as 0; 1; 3; 5; and 7 % to dope membrane. The result showed that higher concentration of the both surfactant resulting higher water flux. The highest water flux is 328,6 L/m2.h with 7% Tween20 addition. Contrary to the rejection performance of BSA, the result showed decreasing rejection value in higher surfactant addition. The BSA rejection by PVC original membrane is about 97%, after addition of surfactant additives the rejection value decreased up to around 86-87,5% at 7% Tween20 and Tween80.
In this study, pluronic was developed to improve the performance of PVC membranes. PF127 is a copolymer with two segments—hydrophilic and hydrophobic (Figure 2). The polyethylene oxide (PEO) segment of PF127 improves the hydrophilic characteristics of the membrane’s surface, while polypropylene oxide, which is hydrophobic, attaches closely to the matrix of the membrane9. The hydrophile–lipophile balance value of PF127 ranges from 18 to 2312. In this study, PF127 is used as an additive to improve the performance of a PVC membrane.
Polyvinyl chloride (PVC) with an average molecule weight of 43,000 Da was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Dimethyl acetate (DMAc) solvent was obtained from Wako, Pure Industries Japan. Distilled water was produced in the laboratory. PF127 was obtained from BASF Co. (Ludwigshafen, Germany). Dextran with an average molecular weight of 10,000 Da, which was used for the rejection test, was bought from Sigma-Aldrich. All chemicals were used directly without previous treatment.
The wet inversion technique was adopted to prepare the membrane using water as a non-solvent coagulation media. PVC with a concentration of 15 wt% and PF127 with concentration of 1, 3, 5 and 7 wt% were dissolved in DMAc to improve the performance of the membrane The solution was stirred with a magnetic stirrer at 200 rpm until it was homogeneous. The homogeneous membrane solution was left for 24 hours at room temperature to completely discharge the air bubbles. The solution was then framed on a glass plate using an automatically adjustable applicator (YBA-3, Yoshimitsu, Japan) at a thickness of 450 µm. The glass plate containing membrane was dipped in a coagulation bath of distilled water. The de-mixing process between the DMAc solvent and non-solvent distilled water solidified the membrane and separated it from the glass plate.
Membrane morphology was observed using scanning electron microscopy (SEM) (Hitachi Co, S-800) with an accelerating voltage of 15 kV. To obtain a clean and dry sample, about 1 cm2 of the membrane sample was freeze-dried (Eyela FD-1000, Japan) for 24 hours. To ensure that the structure of the membrane was not damaged, the membrane sample was ruptured in liquid nitrogen. Next, the membrane sample was mounted on the metal module, followed by the coating process with Pt/Pd sputtering. The coated sample was inserted into the SEM apparatus, and the photo was captured at 5.0 kV. Three images was collected for each Three images each were collected for PVC membranes containing 0, 3, 5 and 7% P127.
The permeability of water and solute rejection were tested with the module of dead-end filtration (Advantec, UHP-43K, Japan). The transmembrane pressure was regulated at a pressure of 0.5 atm. The effective membrane surface area that passed by water was 0.023 m2. The testing of water permeability was conducted four times, and the average values was taken to determine the final permeability. The permeability coefficient of pure water was counted using Equation 1.
Where Lp = permeability coefficient (L/m2.jam.atm); V = permeate volume (L); A = membrane surface area (m2); and Δp = pressure change (atm).
A dextran solution of 100 p.p.m. was prepared to analyze the rejection efficiency. Equation 2 was used to calculate the rejection value of the fabricated membrane.
Where R = rejection coefficient; Cp = permeate concentration; and Cf = concentration of feed.
The hydrophilicity of the surface of the membrane was measured using a water contact angle meter (Kyowa Kaiwenkagaku, Saitama, Japan, CA-A). The contact angle is the angle formed between the surface of the test material and the pure water dropped onto the surface of the membrane13. Each sample was measured 10 times, and the average value of the measurement was the value of the water contact angle of the membrane sample.
To study the effect of the blending of pluronic additives on the toughness of the PVC/ PF127 membrane, a membrane shrinkage test was performed. The wet cast of membrane at each PF127 concentration was dried in the oven for 24 hours at 80°C. The shrinkage of the membrane was calculated using Equation (3).
Where L0 = length of wet membrane (cm) and L1 = length of dried membrane (cm).
The results of the SEM analysis of the cross-sections of all the membranes are shown in Figure 3. The transverse part of the original PVC membrane has an asymmetric structure consisting of a thick, dense structure in the top layer and a finger-like structure in the center path of the cross-sectional area. The formation of a membrane structure fabricated using the wet inversion technique happens during coagulation, in which the DMAc solvent is leached out of the matrix of the dope solution and water as a non-solvent diffuses into the membrane. This phenomenon causes the formation of membrane pores and a finger-like macrovoid structure14. The structure of the PVC membrane changes after the addition of PF127 as the additive in forming the membrane pores9.
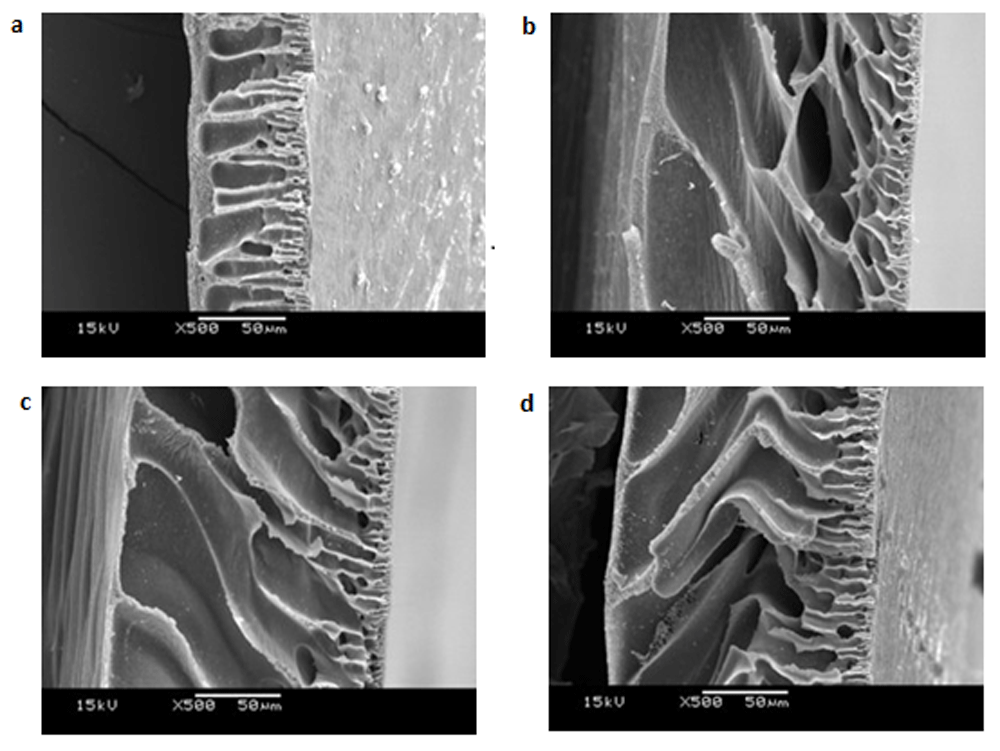
Morphology structure of PVC membrane on transverse part at PF127 concentrations of 0% (a); 3% (b); 5% (c); and 7% (d). Each image is representative of n=3 scanning electron microscopy images.
As shown in Figure 3a, the original PVC membrane has an upper layer that is thicker than the upper layer structure after the addition of PF127. The exchange of solvent from the polymer solution to the coagulation bath occur slowly in the case of the original PVC membrane, and contribute to the formation of a thick upper layer that is larger than in the other systems15. After the addition of PF127, the membrane surface becomes more hydrophilic and the affinity between the casting solution and water increases, so the polymer solution will attract more water and the diffusion process of water into the polymer matrix will be faster16. As a consequence of this mechanism, large macrovoids and a thin upper layer are formed. As can be seen in crosssection layer, the finger like structure forms at PVC membrane with 3wt% of PF127 is dominate a half of the crossection area of the membrane. While, in case of the addition of 5 and 7wt% of PF127, the finger-like structure formed in all cross-section area of the membrane. In other words, the increase in the PF127 concentration results in larger pores and a thinner upper layer of the membrane.
The measurement of the water contact angle is the simplest way to identify the degree of hydrophilicity and hydrophobicity of the membrane15. The hydrophilicity of the membrane, as measured by the water contact angle meter, is shown in Figure 4. The addition of PF127 is proven to improve the hydrophilicity of the membrane, as indicated by the decrease in the water contact angle. The existing PEO segment contained in the PF127 on the membrane surface contributed to the improvement in the membrane hydrophilicity. The hydrophilic nature of PEO chain in Pluronic increased the diffusion rate of non-solvent during membrane solidification. The rapid diffusion of nonsolvent promoted instantaneous demixing, which enhanced macrovoid formation. It has been reported that a rapid precipitation caused by the hydrophilicity of the additive results in higher surface porosity and more porous sublayer, leading to a higher water permeation17. A number of studies on the mechanism of the decreased water contact angle of various membrane modifications with Pluronic have been reported by researchers17–20. The most hydrophilic membrane surface obtained in this study was found in the PVC membrane with the addition of 7wt% additive, with a contact angle of 67.2°.
The water permeability and rejection profile of the original PVC and PVC blend membrane are shown in Figure 5. The original PVC membrane has a water permeability of about 0.616 l/m2.h.atm. After the addition of 1 wt% of PF127 to the dope solution additive, the water permeability increases significantly. The PEO chain in PF127 increases the pore size of membrane. Therefore, the amount of water that passes through the membrane is higher than that of the membrane without the polymeric additive. The change in the bottom layer structure of the PVC blend membrane is also evidence of the increased water permeability (Figure 3). As reported by many authors, the addition of an appropriate amount of hydrophilic polymer to the dope solution might enhance the membrane pores7,15,21,22, and, consequently, high permeation would be obtained. In this work, the highest water permeability reached 45.618l/m2.h.atm, which was obtained in the case of the blend membrane with the PF127 concentration of 7wt%.

Figure 5 also shows the rejection efficiency of the dextran solution. The original PVC membrane is able to reject the dextran molecules by up to 100%. The addition of PF127 at a high concentration causes a decline in the rejection efficiency. The solution sample for the rejection experiment was prepared by dissolving a low molecular weight of dextran (i.e., 10.000 Da). This may be the reason for the reduction of rejection efficiency at high concentration of additive in this work. To achieve the best performance for permeation and selectivity, the optimization of the polymer solution could be designed by changing the relative composition of the PVC and the PF127.
In reference to the separation industry, membranes are expected to sustain in a wide range of temperature conditions. To determine the resistance of PVC/PF127 membranes in high-temperature conditions, a shrinkage test was performed by drying the membranes at 80°C; the results are shown in Table 1. The original PVC membrane did not suffer significant shrinkage after exposure to a temperature of 80°C, nor did the blending of PF127 into a polymer solution contribute seriously to the shrinkage of the PVC membrane. As shown in Table 1, an increase in the additive concentration of up to 7 wt% only has a small impact on the decrease in membrane size. PVC is one of the most widely used polymers in UF membranes, owing to its excellent physical and chemical properties. PVC polymer has a melting point of 212°C, and material fabricated from this polymer can only be degraded at high temperature23.
| PF127 in PVC membrane (wt%) | Shrinkage (%) |
|---|---|
| 0 | 0.67 |
| 1 | 2.07 |
| 3 | 3.33 |
| 5 | 5.00 |
| 7 | 9.33 |
The fabrication of PVC membrane with PF127 as an additive has been performed in this work. The characteristics and performance of the membrane have been analyzed in terms of the morphology, hydrophilic or hydrophobic properties, water permeability, and solute rejection, as well as membrane shrinkage. From this recent study, it can be concluded as follows:
1. Morphological analysis using SEM shows the increasing the membrane porosity after addition of PF127.
2. The water permeability of PVC membrane increases from 0.61 to 45.61 l/m2.hr.atm after addition of 7wt% PF127. However, the optimum filtration result, water permeability and rejection are found on the membrane with 3wt% addition PF127. It reach 20,2 L/m2.h for permeability and 68,66% for solute rejection.
3. The number and length of the macrovoid structure in the center section of the membrane increased and change after the presence of PF127
4. Regarding the water contact angle observation, it is found that the hydrophilicity of the membrane improves as the proportion of PF127 is increased.
5. On the basis of the results of the shrinkage test, it can be concluded that the PVC membrane obtained in this research is able to withstand extreme temperature conditions of up to 80°C.
6. Regarding the experimental results, it can be concluded that PF127 succeeded in improving hydrophilic properties, filtration performance, and maintaining the stability of the membrane. Thus, the PVC-FP127 is useful to be applied in the water treatment industry.
Dataset 1. Raw data for water permeability and solute rejection. DOI: 10.5256/f1000research.15077.d20640124.
Dataset 2. Raw data for water contact angle. DOI: 10.5256/f1000research.15077.d20640225.
Dataset 3. Raw data for membrane shrinkage test. DOI: 10.5256/f1000research.15077.d20640326.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Membrane technology, Production of polymeric membranes
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Bilad M, Guillen-Burrieza E, Mavukkandy M, Al Marzooqi F, et al.: Shrinkage, defect and membrane distillation performance of composite PVDF membranes. Desalination. 2015; 376: 62-72 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Membrane technology, Production of polymeric membranes
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 10 Jul 18 |
read | read |
|
Version 1 12 Jun 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)