Keywords
Streptococcus pneumoniae, serotypes, Nairobi, Quellung reaction, Optochin test, Bile solubility
Streptococcus pneumoniae, serotypes, Nairobi, Quellung reaction, Optochin test, Bile solubility
Streptococcus pneumoniae (SPn) is a highly invasive, Gram-positive, extracellular bacterial pathogen (Mitchell & Mitchell, 2010). It is a major cause of morbidity and mortality globally, causing more deaths than any other infectious disease (Jones et al., 2010). SPn is classified into serogroups (denoted by numbers and letters, e.g. 18c, 23f) (Kellogg et al., 2001). There are over 90 known serotypes and the prevalence of different serotypes varies by regions of the world (Hackel et al., 2013). Different serotypes exhibit differing potentials to cause disease and may cause different syndromes in different age groups (Harboe et al., 2009).
Some strains also have a greater potential to develop antibiotic resistance (Song et al., 2012). The 13 most common serotypes of SPn pneumonia cause 80–93% of serious pneumococcal disease in children (Johnson et al., 2010). According to the World Health Organization (WHO) and UNICEF Global Action Plan for the Prevention and Control of Pneumonia, pneumonia kills more children than any other illness in the world (WHO & UNICEF, 2009). Given the high burden of under-five mortality associated with pneumonia, control efforts are critical to achieving Sustainable Development Goal 3 (Colglazier, 2015).
WHO and UNICEF estimates indicate that over 800,000 children under 5 years of age die from pneumococcal disease each year (O’Brien et al., 2009). In Kenya, an estimated one in every five children under 5 years of age dies from this disease every year (WHO, 2013).
SPn vaccines protect against several severe forms of pneumococcal disease, such as meningitis, pneumonia and bacteremia (Feldman & Anderson, 2014). These vaccines will not protect against these conditions if they are caused by agents other than SPn or from other strains of SPn that are not contained in the vaccine (Moffitt & Malley, 2011). The 10-valent pneumococcal conjugate vaccine (PCV10) was introduced into the Kenya Expanded Program on Immunization (KEPI) in February 2011 with a 2+1 schedule (at 6, 10, 14 weeks) without catch-up vaccinations (Hammitt et al., 2014). The vaccine covers 1, 4, 5, 6b, 7f, 9V, 14, 18c, 19f and 23f SPn serotypes.
Currently over 90 different serotypes have been identified, six of them very recently (Weinberger et al., 2011). Various SPn serotypes with antigenic similarities are classified under the same groups (9A, 9L, 9N and 9V) while those lacking antigenic similarities are given numbers only (1, 2, 3, 4 and 5). The degree of interaction (cross-reactivity) between various SPn groups may vary. For instance, serotypes 6A and 6B have identical chemical composition except for one of the bonds between two sugars yet they are highly cross-reactive but serotypes 19F and 19A are less reactive.
Pneumococcal conjugate (PCVs) and polysaccharide (PPVs) vaccines are designed according their virulence mechanisms and how they generally interact with the human immune system (Castañeda-Orjuela et al., 2012). The WHO has advised that all children ≤5 years should be immunized against pneumococcal disease and continuous surveillance done to keep out the disease especially in the developing world (Vandenbos et al., 2013). The need for continuous surveillance has been exacerbated by the acute emergence of multi-drug resistant SPn strains and escalated child mortality and morbidity due to pneumococcal disease, despite the availability of PCVs and PPVs. (Väkeväinen et al., 2010). This study therefore sought to establish the SPn serotypes among vaccinated and unvaccinated children ≤5 years of age in Nairobi County, Kenya.
This study was conducted among children ≤5 years attending the outpatient department of Gertrude's Children's Hospital, Muthaiga, and its satellite clinic in Githongoro, Nairobi County between May 2017 and February 2018. Subjects were clinically assessed by a physician and those who presented with pneumococcal disease symptoms recommended to the study nurse for recruitment. Gertrude's Children’s Hospital is the largest standalone health care facility specializing in pediatric care in East and Central Africa. The hospital is accredited by the Joint Commission on International Accreditation (JCIA). SPn isolation and stocking was done at Gertrude's Children’s Hospital Main Laboratory and capsular serotyping done at KEMRI Wellcome Trust, Kilifi, Kenya.
This was a descriptive cross-sectional study. Streptococcus pneumoniae serotype epidemiology among PCV-10 vaccinated and unvaccinated children between 6 months and 5 years of age was measured. Children who had no history of any chronic disease and whose parents or legal guardians consented to the study were systematically recruited. Children whose parents or legal guardians declined to give consent and those with any known immunosuppressive conditions were excluded from the study.
To determine the minimum sample size, the formula developed by Chow et al. (2007) was used, with a prevalence rate of 16% (Agweyu et al., 2014).
Where n= desired minimal sample size; z= standard normal deviation (1.96, from the tailed normal table); p̂= prevalence rate; and m= the desired degree of accuracy at a 95% confidence level of 0.05. This gave a sample size of 206.
Nasopharyngeal swabs were per nasally collected using Copan flocked swabs and temporarily suspended in Armies medium for transportation to the main laboratory. Each swab was inoculated onto a selective gentamicin with 5% sheep blood agar (BA) plate. All swabs were plated within 24 h of collection. The plates were incubated at 37°C in a 5% CO2 atmosphere and examined at 16–24 h and then again at 40–48 h for growth of SPn. Isolates were identified as SPn by colony morphology (Mucoid, draughtsman appearance, α-haemolysis) and susceptibility to optochin (positive, ≥14 mm zone of inhibition; negative, <14 mm zone of inhibition). Plates with colonies akin to SPn morphological features but with optochin clearance zones below 14 mm were further subjected to solubility in bile salts (positive, bile soluble; negative, bile insoluble).
The isolation of a single colony indicated carriage. Single colonies were picked using sterile inoculating loops and evenly plated on BA. After 24–48 h, enough inoculum was stocked in brain heart infusion (BHI) agar with 5% sheep blood (Ultralab East Africa, Ltd), gently vortexed and stored at –70°C for serotyping.
Capsular serotyping was done using the Quellung reaction test. Frozen vials containing SPn stocks stored at -70°C were thawed at room temperature for about 30 min. Next, 10 µl of the stored SPn cells were suspended in 50 µl PBS and gently vortexed. Subsequently, 10 µl of the suspended cells were added on to a glass slide and mixed with 10 µl pooled antisera (Statens Serum Institute, cat. No. 16744). The glass slide was swirled gently while observing for any agglutination reaction until a positive reaction was observed with various pooled antisera. The process was repeated with individual groups under various antisera pools.
After that, 10 µl of the suspended cells in PBS were added to a glass slide and mixed with various SPn serotype-specific antisera included in the antisera pools that gave a positive reaction. This was done until a positive reaction with the particular serotype specific antisera was observed. Those serotypes that did not belong to any pool were typed directly until a positive agglutination reaction was observed. The cells/PBS/serotype-specific antisera mixture on the glass slide were covered with a cover slip and observed under a phase contrast microscope with a ×100 objective lens with oil emulsion.
Out of n=206 (100%) of the subjects sampled, n=97 (47.1%) were male and n=109 (52.9%) were female. In total, 68 (33.0%) of the children studied were within the age bracket of 6–12 months, 47 (22.8%) were between the ages of 13–24 months, 46 (22.3%) were between the ages of 25–36 months, 17 (8.3%) were between the ages of 37 and 48 months and 28 (13.6%) were between the ages of 49 and 60 months. Out of the total number of subjects (n=206) sampled, 20.39% (n=42) were found to be carriers of SPn; 52% (n=22) of the SPn carriers had received the recommended dose of PCV-10 immunization, while 48% (n=20) had not. All isolates (n=42; 20% of subjects) contained non-vaccine-type SPn serotypes, while n=1 (0.49% of the subjects) of the serotypes were untypeable (Table 1). In total, 18 different SPn serotypes were found in this population. They include: 28F (8 instances), 6A (5 instances), 3 (4 instances), 23B (3 instances), 20 (3 instances), 23A (3 instances), 19B (2 instances), 17F (2 instances), 7C (2 instances), 11A (2 instances), 35F (1 instance), 15B (1 instance), untypeable (1 instance), 48 (1 instance), 35B (1 instance), 21 (1 instance), 39 (1 instance) and 13 (1 instance).
The percentage of SPn carriage status among PCV-10 vaccinated and unvaccinated children is shown.
Various serotypes were found to be prevalent in different age groups. For instance, out of the 42 serotypes found, 9 (23.53%) were prevalent among children at 6–12 months of age (n=16). They include: 28F (4 instances), 11A (2 instances), 23A (2 instances), 3 (2 instances), 6A (2 instances), 17F (1 instance), 35F (1 instance), 7C (1 instance) and untypeable (1 instance). There were 7 (16.67%) serotypes prevalent among children at 13–24 months (n=8), including: 20 (2 instances), 21 (1 instance), 39 (1 instance), 28F (1 instance), 35B (1 instance), 17F (1 instance) and 13 (1 instance). There were 8 (19%) serotypes found among children of 25–36 months of age (n=12), including: 23B (3 instances), 19B (2 instances), 3 (2 instances), 20 (1 instance), 28F (1 instance), 7C (1 instance), 23A (1 instance) and 48 (1 instance). There were 3 (7%) serotypes prevalent among children at 37–48 months old (n=4), including: 6A (2 instances), 15B (1 instance) and 28F (1 instance).
There were 2 (4.76% of the total) serotypes prevalent among children at 49–60 months (n=2): 6A (1 instance) and 28F (1 instance) (Table 2). Out of the 42 isolates (found in 20.39% of subjects), serotype 28F was the most prevalent (3.88% of the total), followed by 6A (2.43%), 3 (1.94%) and 20, 23A and 23B all at 1.46% (n=3). Each of the serotypes 7C, 11A, 17F and 19B represented 0.97% (n=2) of the total serotypes, while serotypes: 13, 21, 39, untypeable, 48, 15B, 35B and 35F represented 0.49% (n=1) each of the total serotypes found (Figure 1 and Figure 2). In total 51% (n=106) of the total sampled subjects were confirmed to have received a full dose of the PCV-10 vaccination as per the recommended schedule of immunization at 6, 10 and 14 weeks. Approximately 11% (n=12) of the immunized children were carriers of SPn in their nasopharyngeal region; 10% (n=10) of the non-immunized group were also carriers (Table 3). Serotypes 28F (5 instances), 23A (3 instances), 6A (3 instances), 17F (2 instances), 11A (1 instance), 3 (1 instance), 35F (1 instance), 48 (1 instance), 13 (1 instance), 35 (1 instance) and 7C (1 instance) were prevalent among the 9.71% (n=20) of the total sample group that had not received PCV-10 immunization. Serotypes 3 (3 instances), 28F (3 instances), 23B (3 instances), 20 (3 instances), 19B (2 instances), 6A (2 instances), 21 (1 instance), 11A (1 instance), 7C (1 instance), untypeable (1 instance), 15B (1 instance) and 39 (1 instance) were prevalent among the 10.68% (n=22) of the total sample group that received immunization (Table 4).
The SPn serotypes as found among PCV-10 vaccinated and unvaccinated children of varying age groups is shown.
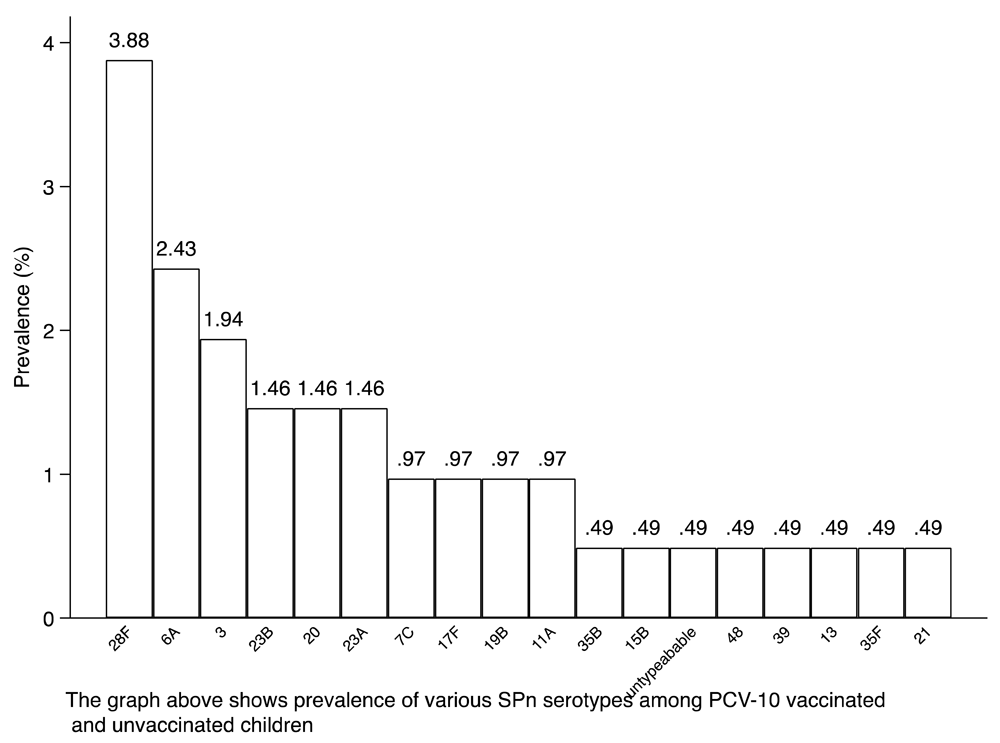
This figure shows the prevalence of various SPn serotypes among PCV-10-vaccinated and –unvaccinated children.
The percentage prevalence of SPn serotypes among PCV-10 vaccinated and unvaccinated children is shown.
| Child immunization status | SPn | n | % |
|---|---|---|---|
| Immunized | NGR | 84 | 40.78 |
| SPn | 22 | 10.68 | |
| Not immunized | NGR | 80 | 38.83 |
| SPn | 20 | 9.71 | |
| Total | N/A | 206 | N/A |
This study found that 20.39% of all children studied, from both the PCV-10 vaccinated and unvaccinated groups, were carriers of SPn. While this is a significant reduction from the pre-vaccine era, it is still high compared to malaria, diarrhea and HIV/AIDS (Feikin et al., 2010). In total, n=41 of the serotypes found were non-vaccine type (in 19.90% of the subjects), with one additional untypeable serotype. This is a very important finding as it explains the high level of child morbidity and mortality due to pneumococcal disease despite the availability of PCV-10.
While these findings agree partially with those of Jacobs et al. (2008), where a significant decrease in the vaccine type SPn serotypes found in isolates was observed, a 97.6% (n=41) decrease is, at the very least, surprising. This trend may be attributed to the increased level of antimicrobial misuse by a greater percentage of the study population (Domenech de Cellès et al., 2011). 10-valent pneumococcal conjugate vaccine contains 10 different serotypes, which include: 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F (Slotved et al., 2016). None of these 10 serotypes was found in the study population yet this is the vaccine currently included in KEPI, targeting the same population.
Streptococcus pneumoniae carriage decreased with age as 11.65% (n=24) were obtained from children aged between 6–24 months and 8.74% (n=18) from children >24 months. The study results demonstrated a linear relationship between child age and SPn carriage. A similar study done elsewhere reported findings that partly agree with this and partly disagrees (Hill et al., 2008). The former being attributable to development of SPn-specific IgG antibodies due to vaccination and during that window before most children start attending school (Corscadden et al., 2013). Unlike findings from a study by de Paz et al. (2015), serotype 28F was the most prevalent and was present in all five age groups profiled. This is a likely scenario of serotype replacement as SPn attempts to evade the action of the immune system and eventually shares the resistant genes within the microbial community, especially in the nasopharyngeal region (Donati et al., 2010).
Serotypes 28F, 6A, 11A, 3 and 7C were prevalent in both vaccinated and unvaccinated children, whereas serotypes 23A, 17F, 35F, 48, 13, 35B and 23B, 20, 19B, 21, untypeable, 15B, 39 were found among unvaccinated and vaccinated groups respectively. There exists different antigenic features between and within various strains of SPn (Song et al., 2012). While the majority, if not all, pneumococcal serotypes are capable of causing disease, the frequency with which they are isolated varies (Kalin, 1998). In this case, vaccination would only be partially effective and, if so, due to inter-strain antigenic characteristics.
While trying to evade the action of the immune system, SPn has a tendency to exchange resistant genes and other antigenic correlates at the nasopharyngeal region (Johnston et al., 2014). Resistance to antimicrobial agents is occasioned by among other factors, misuse of antibiotics (Dinsbach, 2012). This is largely due to a lack of properly enforced antibiotic use regulations by the authorities.
Dataset 1. List of basic demographic information for each subject, with the size of the optochin clearance zone and serotype of Streptococcus pneumoniae, if found. DOI: http://doi.org/10.5256/f1000research.14387.d207458 (Walekhwa et al., 2018).
The study was in part funded by the National Commission of Science Technology & Innovation (NACOSTI) Kenya. Ref Nos. NACOSTI/RCD/ST & I/7TH CALL/PhD/148.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Special thanks to Prof. Anthony Scott, Dr. Claire Gordon and Ms. Angela Karani of Kemri Wellcome Trust, Kilifi for their professional input. The laboratory and technical team: Ann Karanu, Elizabeth Mbithe, Shadrack Mutua and Alfred Too. Your technical input was amazing! The Gertrude's Hospital clinical and laboratory team (Linet Okose, Barasa Kasili, Charles Muia, Lilive Njagi, Carolyne Thumbi, and Peninah Chege), you made this possible. Thanks to Japheth Wambani Rapando for the review of my work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, et al.: Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group.Vaccine. 2013; 32 (1): 165-79 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Medical microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medicinal parasitology (Malariology), immunoparasitology and medical entomology, antimicrobial activity of plant extracts
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunoparasitology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 31 Jan 19 |
read | ||
|
Version 1 22 Jun 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)