Keywords
Drosophila, fruit fly, paclitaxel, nociception, pain, CIPN
Drosophila, fruit fly, paclitaxel, nociception, pain, CIPN
Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting side effect of many effective cancer treatments (Burton et al., 2007), and can have a lasting impact on the quality of life of cancer survivors (Hausheer et al., 2006 and Shimozuma et al., 2012). A meta-analysis of 31 studies from over 4000 chemotherapy-treated patients revealed that CIPN was prevalent in 68.1% of patients in the first month following chemotherapy, in 60% of patients at 3 months, and in 30% at 6 months or more (Seretny et al., 2014).
Paclitaxel has a potent ability to cause CIPN (Addington & Freimer, 2016; Reyes-Gibby et al., 2009). Derived from the bark of the western yew, Taxus brevifolia, it is an approved and effective treatment against breast, ovarian, lung and Kaposi sarcoma (Chang et al., 1993; Gill et al., 1999; Holmes et al., 1991; McGuire et al., 1989; Wani et al., 1971). Patients treated with paclitaxel experience side effects as early as one to three days following treatment (Lipton et al., 1989; Reyes-Gibby et al., 2009). Common symptoms are hyperalgesia, hypoalgesia, allodynia, tingling, numbness, and shooting pain (Boland et al., 2010). Paclitaxel has a direct effect on Schwann cells, promotes axonal degeneration, and can cause mitochondrial damage (André et al., 2000; Cavaletti et al., 1995; Sahenk et al., 1994), however the molecular mechanisms causing pain are still largely unknown.
While much knowledge has been gained about the genetics of pain from vertebrate systems, high throughput dissection of pain is possible using the fruit fly Drosophila melanogaster (Neely et al., 2010). When challenged with a noxious thermal stimulus, third instar larvae exhibit an aversive escape response that has been utilised to identify conserved genes required for nociception (Babcock et al., 2009; Neely et al., 2010; Tracey et al., 2003). This nociceptive response is a result of activating class IV multidendritic-dendritic arborisation (md-da) sensory neurons at the site of stimulation (Hwang et al., 2007). Previously in Drosophila, paclitaxel has been reported to be toxic in somatic cells, and causes loss of axons in peripheral nerves. (Bhattacharya et al., 2012; Cunha et al., 2001). However, its effects on nociception have not yet been evaluated. Here, we examined the effects of paclitaxel exposure on the fruit fly larval nociception system, and observed a robust and dose-dependent increase in pain perception. This system is amenable to high throughput screening and genetic manipulation, and may help define why chemotherapies such as paclitaxel cause pain.
All flies were reared at 25°C and 65% humidity over a 12-hour light-dark cycle. Six female and two male Canton S Drosophila melanogaster were mated on food medium (5.4% sucrose, 3.6% yeast, 1% agar, 1.2% nipagin, and 0.6% propionic acid) treated with ethanol, 0 µM, 0.1 µM, 0.5 µM, 2.5 µM, 5 µM or 10 µM paclitaxel (Taxol®; Catalog No. A4393) purchased from ApexBio (Houston, USA). A stock of 1000 µM paclitaxel in ethanol was prepared and diluted in food medium accordingly to create the different drug concentrated food. F0 Flies were discarded two days after mating and F1 larvae were left to grow for another three days. On the fifth day, early third instar were collected to assess nociception or dendritic morphology.
For the thermal nociceptive assay (Tracey et al., 2003), distilled water was added to experimental vials to soften the food and release the foraging third instar larvae. The softened, liquid food was then passed through mesh to catch the larvae to be transferred to a 100mm petri dish sprayed with distilled water. The larvae were touched laterally on abdominal segments four to six with a heat probe (soldering iron with narrow tip) set to 42°C or 46°C. The rolling response was measured in seconds with a cut-off of 10 seconds. For each drug concentration, five repeats were performed, with 30–40 larvae per repeat.
Third instar larvae (w;ppk-CD4-tdGFP) were collected, washed, and placed onto a sylgard dissecting plate (Sylgard184 silicone elastomer kit, Dow Corning, Midland, MI). At least 10 larvae of each group were then dissected in HL-3 saline solution (70 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 5 mM HEPES, 115 mM sucrose, 5 mM threalose, 20 mM MgCl2, pH 7.2). Dissected larvae were fixed with 4% paraformaldehyde solution, and then washed in PBS four times. Larvae of the same genotype were then transferred to microscope slides to be mounted using Vectashield (Vector Laboratories, Burlingame, CA).
GFP-expressing class IV md-da sensory neurons were imaged on a Leica DMI-6000 inverted microscope and SP8 Basic Confocal system using 40x oil immersion lens (Leica, Wetzlar, Germany). Class IV neuron at abdominal segment 5 (A5) of each larva was imaged. 488 nm lasers for excitation were used, emission signal was captured at 500–550nm wave length, scanning speed of 400 Hz, zoom factor of 0.7, and gain of 600. Images of Z-stack sections were captured at 1024 × 1024 pixel resolution with section thickness of 0.4 µm. Z-stack images were then rendered into a maximum intensity projection using ImageJ. Total dendritic surface area was quantified using ImageJ. The experiment was conducted in a blinded manner.
Data are presented as mean ± SEM and compared to vehicle control. Analysis was done using GraphPad Prism 5. Statistical analysis for response time was done using Krustal-Wallis ANOVA, followed by Dunn’s pairwise test for multiple comparisons. Statistical analysis for dendritic area percentage was done using Student’s t-test.
Our goal here was to develop a reproducible paradigm to investigate the effects of paclitaxel on nociception in the fly larvae. Based on previous studies for toxicity (Bhattacharya et al., 2012; Cunha et al., 2001), we selected paclitaxel doses below the lethal limit (Figure 1A), and then tested larval nociception using a heat probe set to a low intensity noxious heat (42°C; Figure 1B), which is mildly nociceptive to fly larvae (Babcock et al., 2009). Our dose-response study revealed 2.5 µM paclitaxel was sufficient to induce significant hyperalgesia, with a maximal hyperalgesia effect observed at 10 µM (Figure 1C). Concentrations higher than 10 µM paclitaxel were 100% lethal (not shown). Interestingly, paclitaxel did not significantly alter heat nociception latency to a 46°C heat stimulus across any of the doses (Figure 1D). Vehicle (ethanol) control and normal (no ethanol) control showed a response time of 5.71 sec (±0.23 SEM; n=173) and 5.62 sec (±0.20 SEM, n=180, not shown), respectively (42°C; Figure 1E). At low concertation’s of 0.1 µM (5.21 sec ± 0.23 SEM; n=150) and 0.5 µM (5.44 sec ± 0.26 SEM; n=131) paclitaxel did not affect response profiles, however, concentrations of 2.5 µM paclitaxel (4.22 sec ± 0.19 SEM; n=180; p<0.001) and higher altered response distribution and significantly enhanced nociceptive latency (42°C; Figure 1E). The fastest latency response was observed at 10 µM paclitaxel (3.84 sec ± 0.24 SEM; n=140; p<0.001) with a 36.6% increase in response time relative to vehicle control (Figure 1C).
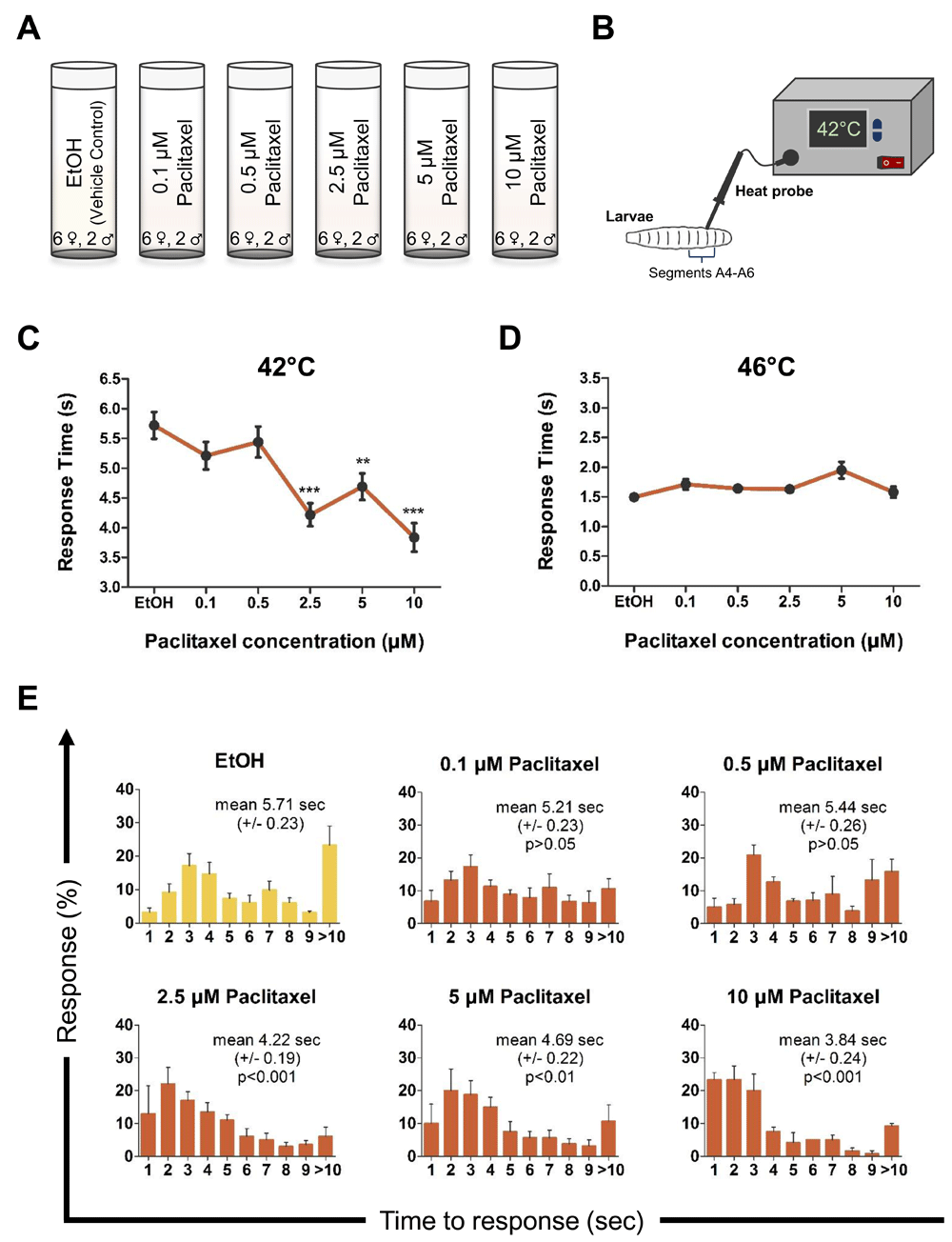
Schematic representation of the A) experimental design and B) thermal nociceptive assay in third instar Drosophila larvae. C–D) Average nociceptive latency (in seconds) in response to a 42°C or 46°C thermal stimulus, respectively. Increased paclitaxel concentration significantly induces heat-hyperalgesia in third instar larvae at 42°C. Note concentrations higher than 10 µM paclitaxel were 100% lethal. E) Percentage response to each time point in seconds to 42°C thermal stimulus. All values represent mean ± SEM. p values were generated using Krustal-Wallis ANOVA, followed by Dunn’s pairwise test for multiple comparisons. Significance is relative to vehicle control. Five repeats were performed for each drug concentration with roughly 30 larvae each (n = 130–180 animals).
To evaluate if paclitaxel exposure caused robust morphological differences in peripheral pain sensing neurons, we fed genetically labeled (w;ppk-CD4-tdGFP) larvae paclitaxel and imaged the sensory neuron structure (Figures 2A–B). Surprisingly, treating larvae with 10 µM paclitaxel did not affect dendritic area percentage compared to vehicle control (Figure C) despite significantly enhancing nociceptive sensitivity (not shown). Thus we establish that paclitaxel sensitizes larvae to heat pain via enhancing sensory neuron or higher order nociception, and not via inducing overt morphological changes.
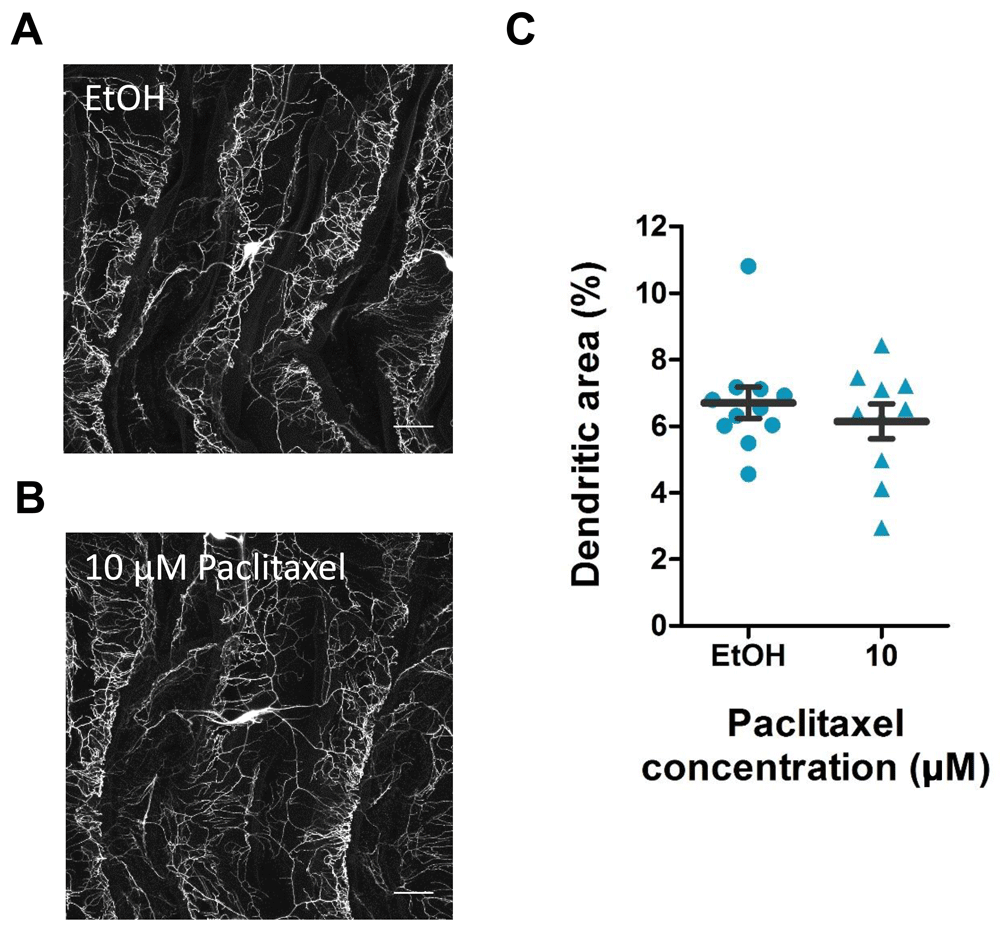
A–B) Confocal images of third instar ppk-CD4-tdGFP larvae following (A) vehicle control or (B) 10 µM paclitaxel treatment. Images are of class IV md-da neurons at abdominal segment A5. Images are at 40x magnification and 0.7 zoom factor. Scale bar represents 40 µm. C) Quantification of dendritic area percentage of class IV md-da sensory neurons. Values represent mean ± SEM (n = 10–11 animals).
Many approved and effective anti-cancer therapeutic agents cause severe pain as a side effect. This can limit our ability to eradicate tumors, and often leaves cancer patients and survivors in intense, and untreatable pain. In this study, we describe a simple system to provide a high throughput dissection of the mechanisms involved in paclitaxel-induced pain. By combining these techniques with genomic approaches to identify regulators of chemotherapy pain, we can better understand how the pain arises, and potentially avoid these severe side effects while more effectively targeting the underlying disease.
Dataset 1: Larval response time in seconds to 42°C heat stimulus. Paclitaxel fed larvae were touched with a 42°C heat probe and their response time was measured in seconds with a cut-off of 10 seconds. Different treatments were tested: food control, ethanol control, 0.1 µM, 0.5 µM, 2.5 µM, 5 µM, and 10 µM paclitaxel. Five repeats were performed (n = 130 - 180). DOI, 10.5256/f1000research.13581.d191022 (Hamoudi et al., 2018a).
Dataset 2: Larval response time in seconds to 46°C heat stimulus. Paclitaxel fed larvae were touched with a 46°C heat probe and their response time was measured in seconds with a cut-off of 10 seconds. Different treatments were tested: food control, ethanol control, 0.1 µM, 0.5 µM, 2.5 µM, 5 µM, and 10 µM paclitaxel. Five repeats were performed (n = 130 - 180). DOI, 10.5256/f1000research.13581.d191023 (Hamoudi et al., 2018b).
Dataset 3: Dendritic morphology of third instar ppk-CD4-tdGFP. Confocal images of vehicle control and 0.1 µM paclitaxel treated larvae. Images represent class IV md-da neurons at abdominal segment A5. Images are at 40x magnification and 0.7 zoom factor. Scale bar represents 40 µm. DOI, 10.5256/f1000research.13581.d222138 (Hamoudi et al., 2018c).
This work was supported in part through NHMRC project grants APP1026310, APP1029672, APP1028887, APP1046090, APP1042416, APP1086851, and by a NHMRC career development fellowship II CDF1111940.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 16 Oct 18 |
read | ||
|
Version 1 23 Jan 18 |
read | read | |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)