Keywords
Callus formation, purple sweet potato, drought tolerance, Polyethylene Glycol, in vitro selection
This article is included in the Agriculture, Food and Nutrition gateway.
This article is included in the ICTROPS 2018 collection.
Callus formation, purple sweet potato, drought tolerance, Polyethylene Glycol, in vitro selection
Drought stress is a major limiting factor in increasing the production of several important crops in a large number of regions in Indonesia. The effect of drought stress on plant growth is largely determined by the amount of stress the plant is exposed to and the growth phase the plant was in during drought stress. In addition, drought stress causes inhibition of plant growth and plant roots1,2 and as a consequence, plants will grow slowly3 and their productivity is severely reduced4. Development of cultivated plants tolerant to drought stress, including sweet potato, is an important approach to solving water shortage issues5.
In vitro selection is a breeding strategy widely used to produce new variant plants that are resistant or tolerant to disease, herbicides or extreme environmental stresses, including drought stress6. This method selects genetic variation arising from tissue culture processes especially produced from natural or artificial mutation7. The rapid multiplication of undifferentiated cells during callus formation increases the possibility of natural mutation due to rapid cell division8. Such genetic changes are subsequently selected as useful traits in breeding programs.
Polyethylene glycol (PEG) solution can be used as drought-tolerant selecting agent in soybean9 and other plants. PEG is able to control the decrease of water potential homogeneously, therefore it can mimic the potential of groundwater10. The long-term use of PEG will not cause cell damage, because PEG has molecular weight of more than 6000 g/mol that cannot be absorbed into plant tissues11. This research attempted to produce new variant of purple sweet potato tolerant to drought stress via in vitro selection using PEG as a selection agent.
Young leaves and petioles from previous in vitro-generated plants grown using standard Murishage and Skoog (MS) medium12 were used as explants. The intact leaf were sliced into 1 cm2 of lamina and 1 cm length of petiole. The explants were then inoculated to the MS containing plant growth regulator of 2,4-dichlorophenoxyacetic acid (2,4-D) (Merck, Cat. No.31518, Germany) combined with 6-benzylaminopurine (BAP, Merck, Cat. No. B3408, Germany) to induce calli. The explants were grown and placed at a sterilized 600 ml bottle containing around 20 ml solidified medium for a month at the culture room at a temperature of 25±2°C. The composition of the treatments were Z1 (MS + 0 mg L-1 2,4-D + 0 mg L-1 BAP), Z2 (MS + 1 mg L-1 2,4-D1 + 0.5 mg L-1 BAP), Z3 (MS + 2 mg L-1 2,4-D1 + 0.5 mg L-1 BAP), Z4 (MS + 2 mg L-1 2,4-D1 + 1 mg L-1 BAP), Z5 (MS + 3 mg L-1 2,4-D1 + 0.5 mg L-1 BAP), Z6 (MS + 3 mg L-1 2,4-D1 + 1 mg L-1 BAP), Z7 (MS + 4 mg L-1 2,4-D1 + 0.5 mg L-1 BAP), and Z8 (MS + 4 mg L-1 2,4-D1 + 1 mg L-1 BAP). All treatments were replicated five times. In total there were two types of explants x eight treatments x five replication x five explants per replication/bottle = 400 experiment units.
The green, fresh and compact calli produced at the first experiment were selected and transferred to MS medium with half the usual level of nutrients containing 0, 5, 10, 15 and 20% PEG as selection agent medium of drought tolerance. All treatments were replicated three times. In total there were five treatments x three replications x four explants per replication/bottle = 60 experiment units. The surviving calli in the PEG selection-agent medium were regenerated in MS medium containing 0, 0.5, 1 or 1.5 mg L-1 BAP to induce shoots and roots. The growth variables during in vitro culture, such as the number of calli induced per treatment, percentage of responsive explants to induce calli, percentage of surviving calli under drought stress simulated by polyethylene glycol, and number of regenerated plants, were observed.
All media applied in the experiment could successfully induce callus formation except the basal medium containing no growth regulator (control). The existence of 2,4-D and BAP in various concentration combination was able to induce callus formation but the number of callus per explant and the percentage of responsive explant were varied (Table 1, Figure 1A, B). The highest number of induced calli per treatment was observed using the Z3 treatment medium. These results were observed using both leaf and petiole (Figure 1A, B). Callus formation was successfully induced in sweet potato using both explants13,14. The use of 2,4-D to induce callus formation is common13,15. Callus formation can be induced by other plant growth regulators such as zeatin, or 1-naphthaleneacetic acid combined with gibberellin (GA3)14,16. These calli emerged and was developed from the mesophyll cells in the leaf and protoplast from the stem or petiole13,14.
Values shown are mean ± standard deviation unless indicated.
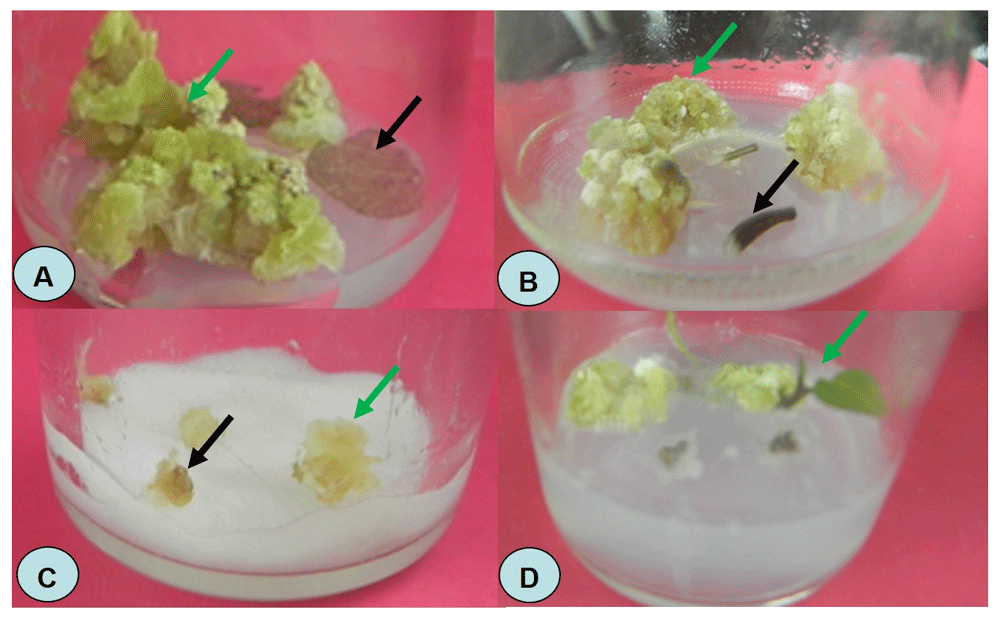
(A) Callus induction from leaf explants (black arrow shows irresponsive explant; green arrow shows the responsive explant). (B) Callus induction from petiole explants (black arrow shows unresponsive explant; green arrow shows the responsive explant). (C) Callus selection for drought tolerant using polyethylene glycol (black arrow shows dead explant; green arrow shows the survived explant). (D) Callus regeneration (green arrow shows the initiated shoot from the callus).
The fresh, green and compact calli produced in the first experiment was transferred to the drought stressed medium simulated by MS medium containing different concentration of PEG to discover the putative drought tolerant calli of purple sweet potato. At least one callus survived in all PEG-containing medium, but the survival rate was varied (Table 2, Figure 1C). The higher the concentration of the PEG in the medium the lower survival rate of the calli in the selection medium. This indicates that drought stress caused by PEG simulation affects callus survivability and can be used as drought-tolerant selection medium, as also reported in other crop plants such as soybean9,17–20, tobacco21, grass22, rice23 and chili24. The putative drought-tolerant plants are screened from regenerated plants derived from the survived calli in the highest concentration of PEG. There was no significant different response between calli derived from the leaf and petiole (Table 2).
| Explant | PEG Concentration (%) | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Leaf | 90.11 | 89.85 | 88.37 | 76.32 | 55.42 |
| Petiole | 96.88 | 86.88 | 81.67 | 76.98 | 57.92 |
The surviving calli grown in medium containing 40% PEG were incubated in the regeneration medium to initiate shoot and/or root. The only medium MS containing 1.5 mg L-1 BA can successfully induce shoot and root growth (Table 3, Figure 1D). This indicates that the calli exposed with high concentration of PEG are still viable to regenerate into whole plant. The results increase the possibility to get a new purple sweet potato variant tolerant to drought stress as also reported in soybean19,20.
| Callus induction medium | Number of regenerated plants | |
|---|---|---|
| Leaf | Petiole | |
| K0 (MS + 0 mg L-1 BAP) | 0 | 0 |
| K1 (MS + 0.5 mg L-1 BAP) | 0 | 0 |
| K2 (MS + 1.0 mg L-1 BAP) | 0 | 0 |
| K3 (MS + 1.5 mg L-1 BAP) | 1 | 4 |
The raw data for calli and explant growth are available on OSF25.
Initial production of putative drought tolerant plants by callus screening using PEG as drought tolerant-selecting agent in purple sweet potato was successfully done in this experiment. Callus was successfully induced in MS medium containing many different combinations of 2,4-D and BAP. Drought-tolerant screening using PEG is generally effective since there was a strong indication that the increase of PEG concentration caused the reduction of the callus survivability. However, the high percentage of surviving calluses at the highest concentration of PEG (40%) may indicate that the drought stress applied in the experiment was not apparently sufficient to induce cell mutation. Therefore, a future experiment using higher concentrations of PEG could provide more tolerant calli and increase genetic mutations, especially with regards to drought tolerance. Genetic evaluation and field experiments using water shortage treatment experiments will be the next investigation to clarify the genetic mutations involved and the stability of the drought-tolerance characteristics.
The raw data on the number of calli formed per bottle for different treatment conditions and subsequent explant growth are available on OSF. DOI: https://doi.org/10.17605/OSF.IO/DEGVY25.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant breeding and plant protection
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 28 Mar 19 |
read | read |
|
Version 1 03 Jan 19 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Should add more literature on suitable PEG concentration to test for drought tolerance in related species.
Materials and Methods:
Adequate.
Results and Discussion:
Adequate.
General comments:
The research idea to develop drought tolerant purple sweet potato ... Continue reading Introduction:
Should add more literature on suitable PEG concentration to test for drought tolerance in related species.
Materials and Methods:
Adequate.
Results and Discussion:
Adequate.
General comments:
The research idea to develop drought tolerant purple sweet potato through in vitro selection by using stress is good and should have supporting funds to do more research until they get the whole plants.
Assoc.Prof. Dr. Duangporn Premjet,
Center of Excellence in Research for Agricultural Biotechnology,
Naresuan University,
Muang, Phitsanulok, 65000, Thailand.
Email: duangpornp@nu.ac.th
Te; +66-55-968761, +66894613972
Should add more literature on suitable PEG concentration to test for drought tolerance in related species.
Materials and Methods:
Adequate.
Results and Discussion:
Adequate.
General comments:
The research idea to develop drought tolerant purple sweet potato through in vitro selection by using stress is good and should have supporting funds to do more research until they get the whole plants.
Assoc.Prof. Dr. Duangporn Premjet,
Center of Excellence in Research for Agricultural Biotechnology,
Naresuan University,
Muang, Phitsanulok, 65000, Thailand.
Email: duangpornp@nu.ac.th
Te; +66-55-968761, +66894613972