Keywords
Morphine, Seizure, Withdrawal, Intrathecal morphine, Opioid, Convulsion
Morphine, Seizure, Withdrawal, Intrathecal morphine, Opioid, Convulsion
Morphine withdrawal is a common medical problem. Patients with morphine withdrawal can present with a variety of symptoms including runny nose, watery eyes, fever, vomiting, nausea, headaches, sweating, chills, muscle aches, diarrhea, high blood pressure, agitation, anxiety, irritability, depression, disorientation, insomnia1. This case report describes a seizure as a clinical complication during an adult male patient’s withdrawal from morphine. The link between opioid withdrawal and seizures is not well studied in adult humans. To the best of our knowledge, only two case series of seven patients and three patients have been reported tying opioid withdrawal to seizures2,3.
A 44-year-old Qatari man known to have persistent back pain admitted to our facility in 2017. He presented with left lower extremity edema that started approximately three to four weeks prior to admission. It was affecting his daily activities like showering and driving. The edema began in his foot and then gradually progressed to his abdomen. A physical examination found soft pitting edema in the left lower limb up to the sacrum posteriorly and to the umbilicus anteriorly. His lower limb showed some redness with no hotness, tenderness, or signs of chronic venous insufficiency. His past surgical history demonstrated multiple back surgeries, as follows; in 1986, he underwent surgical correction and fusion of lumbar scoliosis anteriorly and posteriorly. Additionally, in 1986, he had triple arthrodesis of his right foot. In 1992, he underwent lengthening of his atrophic flail right leg. In 1993, the Harrington rod from the dorsal and lumbar spine was removed. In 2004, he had anterior lumbar interbody fusion with cages at the levels of T10-T11 and T11-T12. In 2008, he underwent revision surgery to extend the anterior instrumentation from T2 to T12. In March of 2014, the patient had intrathecal morphine pump inserted with a morphine infusion rate of 5.75 mg/day.
Upon admission, a Doppler ultrasound scan of his left lower limb revealed no evidence of deep vein thrombosis (Figure 1). The patient was started empirically on amoxicillin-clavulanic acid (875 mg orally every 12 hours for five days), suspecting community-acquired cellulitis as one of the common causes of unilateral lower limb edema, but his edema did not improve. On the fifth day of admission, the patient started to develop new edema on the right leg. A pelvic and abdominal ultrasound scan showed no obvious mass (Figure 2). We then suspected that his intrathecal morphine infusion may be the cause of his peripheral edema, as other common causes were excluded, so the morphine pump was halted, and the pain management team initiated the patient on oral morphine (30 mg) twice daily and tramadol (50 mg) every six hours.
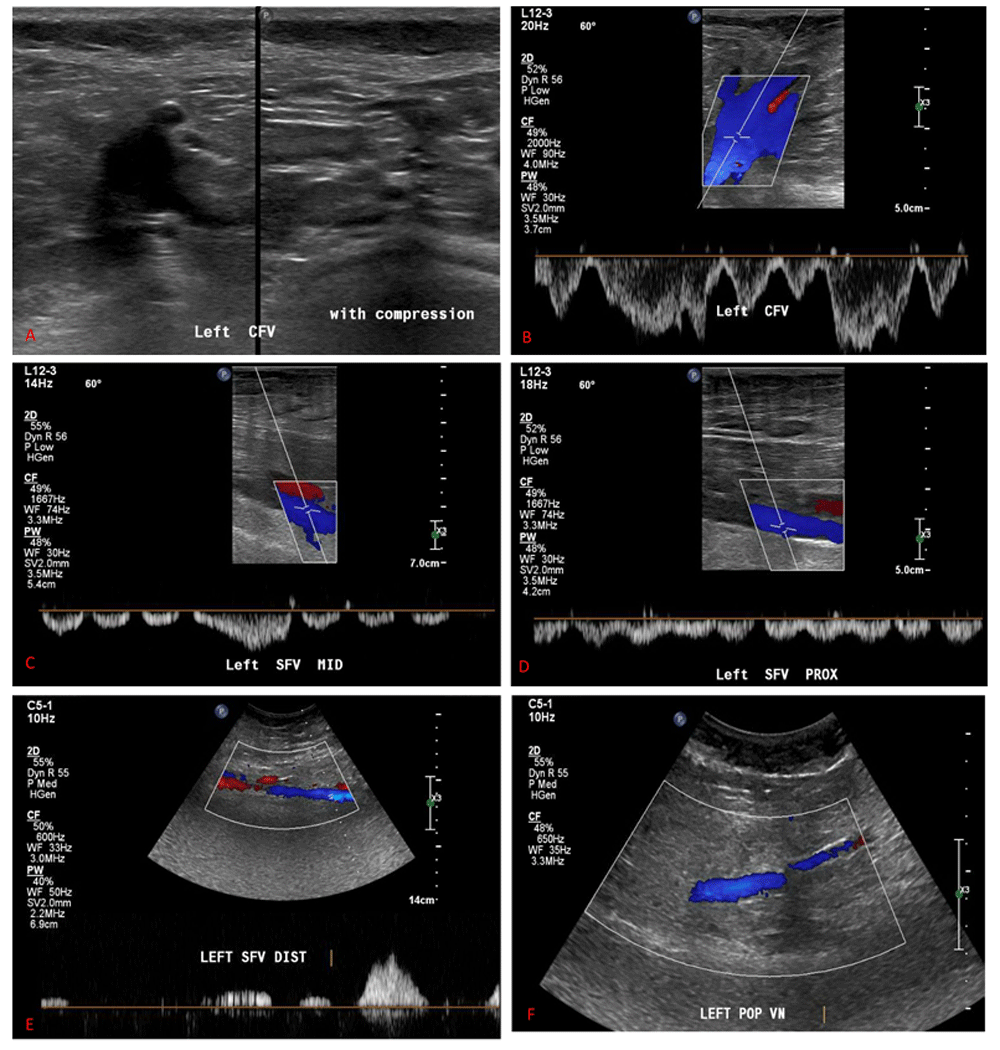
A and B) left common femoral vein; C, D and E) left superficial femoral vein; F) left popliteal.
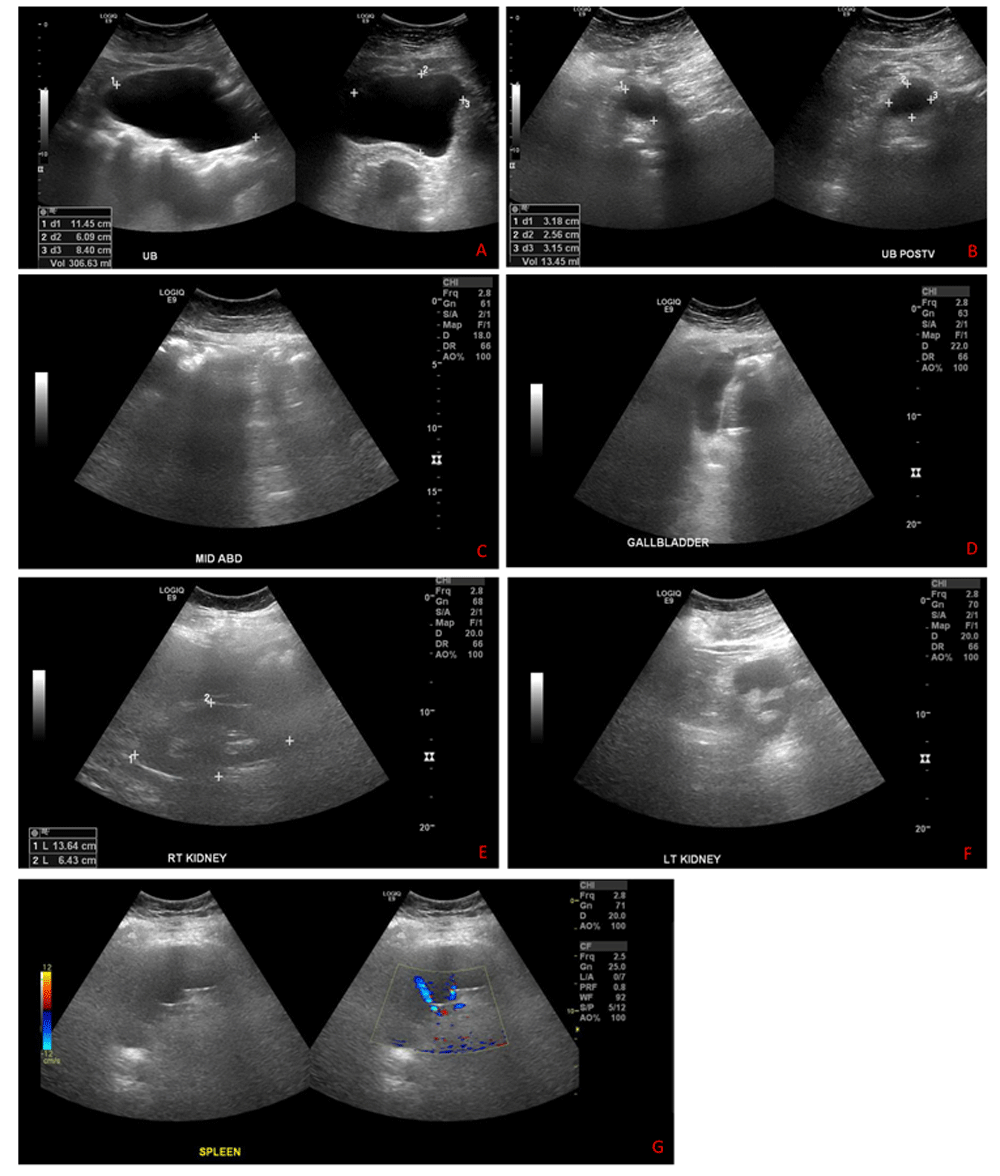
A) urinary bladder; B) urinary bladder postvoid; C) mid abdomen; D) gallbladder; E) right kidney; F) left kidney; G) spleen.
After twelve hours from pump termination, the patient started to convulse. He had three episodes of convulsion over two hours in the form of generalized tonic-clonic convulsion with rolled-up eyes; each episode was preceded by progressive muscle twitches. They were associated with continuous high blood pressure, ranging from 180/100 mmHg to 210/110 mmHg, and profuse sweating. All of the seizure episodes were aborted within a few seconds following the administration of 5mg intravenous diazepam, which was administered one to two minutes after the seizure started. Four hours later, another three seizure episodes occurred. The first was aborted by 5mg intravenous diazepam and the other two episodes required 10mg of intravenous diazepam.
A computed tomography review of the patient’s head was grossly normal and revealed no acute intracranial event (Figure 3). A complete metabolic panel was done and revealed no acute metabolic process or hypoglycemia. The patient’s morphine regimen changed to 5 mg administered intravenously every four hours with oral tramadol (50 mg) every six hours. In the evening, the patient regained full consciousness and no additional seizures occurred.
Upon patient request, the intrathecal morphine pump was restarted. One day after, the patient’s swelling in left lower limb started to increase so the intrathecal morphine pump was stopped, and the patient was started on patient-controlled analgesia fentanyl (50 mcg/hour) and oral methadone (10 mg every six hours). His left- and right-side edema disappeared gradually over seven days and after he regained his baseline functional capacity, he was discharged.
Central nervous system irritability is a known opioid withdrawal sign in neonates4 and is accompanied by seizures in 2% to 11% of cases5,6. While a high degree of cerebral activity and seizure has been reported in rodent model opioid withdrawal studies7, the link between opioid withdrawal and seizures is not well studied in adult humans. To the best of our knowledge, only two case series of seven patients and three patients have been reported tying opioid withdrawal to seizures2,3.
Our patient was not known to be an opioid addict from their history and their opioid risk tool score of one8, so the concurrent use of another known seizure-inducing substance was unlikely. He was receiving an intrathecal dose of morphine which changed to an unequal oral dose of morphine, in addition to tramadol. Seizures are not mentioned in the literature as a known complication of morphine withdrawal, and the patient’s complication may have been caused by severe pain accompanied by inadequate doses of analgesics.
This case illustrates a possible connection between opioid withdrawal and seizure in an adult male patient. Seizure is a life-threating condition; therefore, an awareness of this case is important and further studies are warranted to explore the potential association of opioid withdrawal and seizure.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
References
1. Khanra S, Mahintamani T, Khess CR: Does withdrawal seizure occur in opioid dependence syndrome? A case series.Psychiatry Clin Neurosci. 2015; 69 (4): 238-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Internal medicine, clinical medicine research, neuroscience research
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Addiction Psychiatry, Child Psychiatry, Resilience in wifes of alcoholism,
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Jul 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)